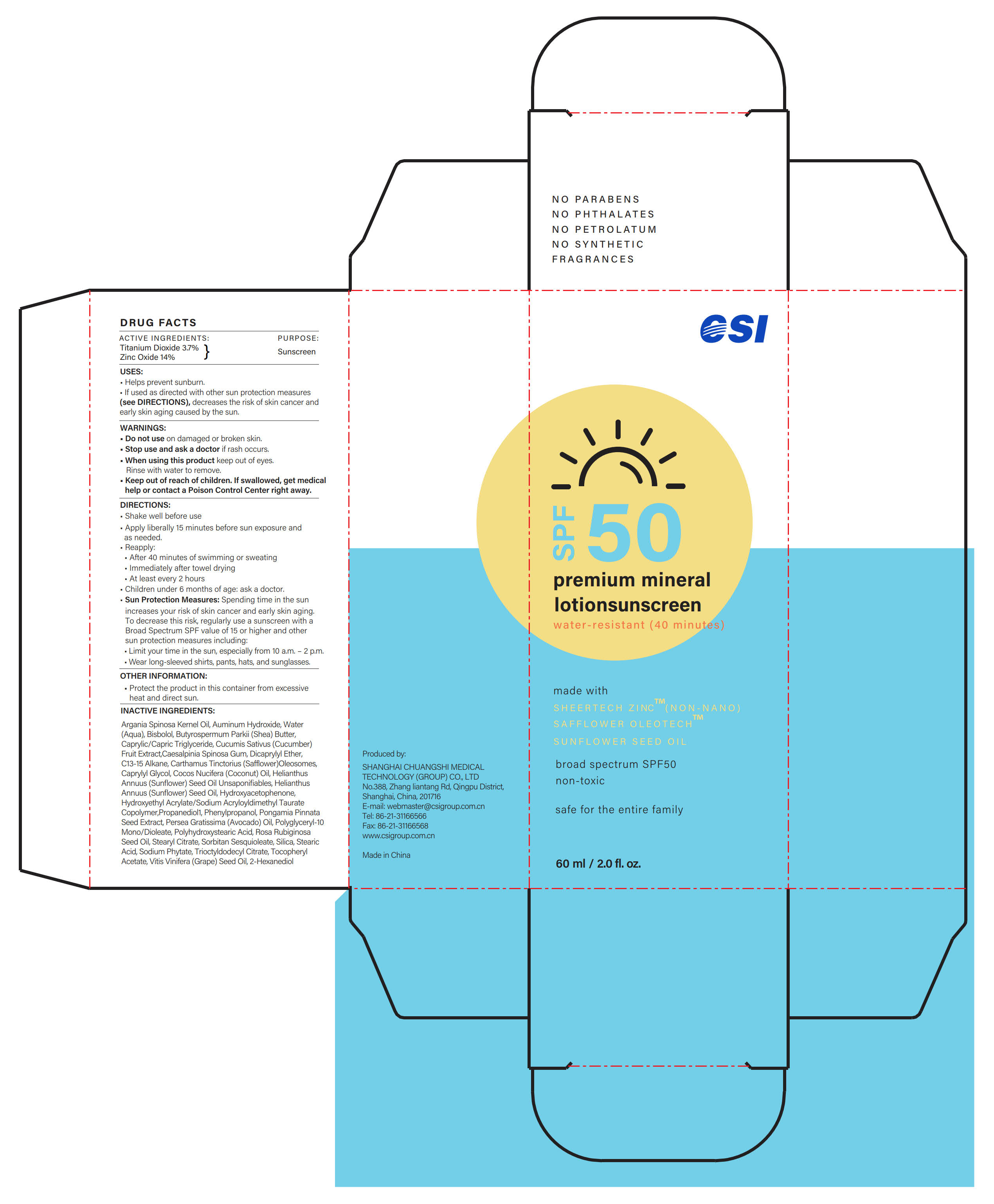
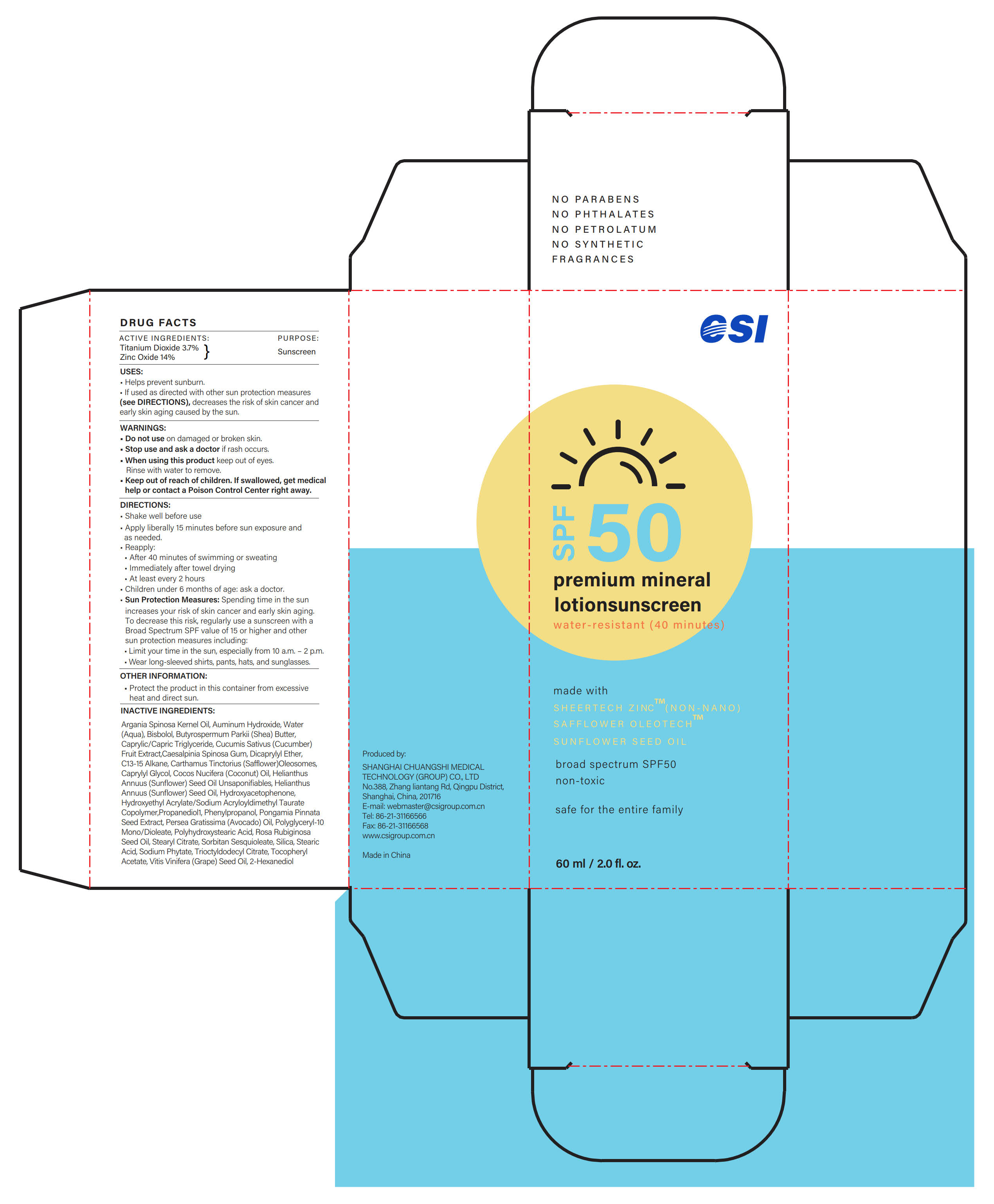
Label: SUNSCREEN-LOTION- zinc oxide, titanium dioxide lotion
- NDC Code(s): 73557-575-03
- Packager: Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
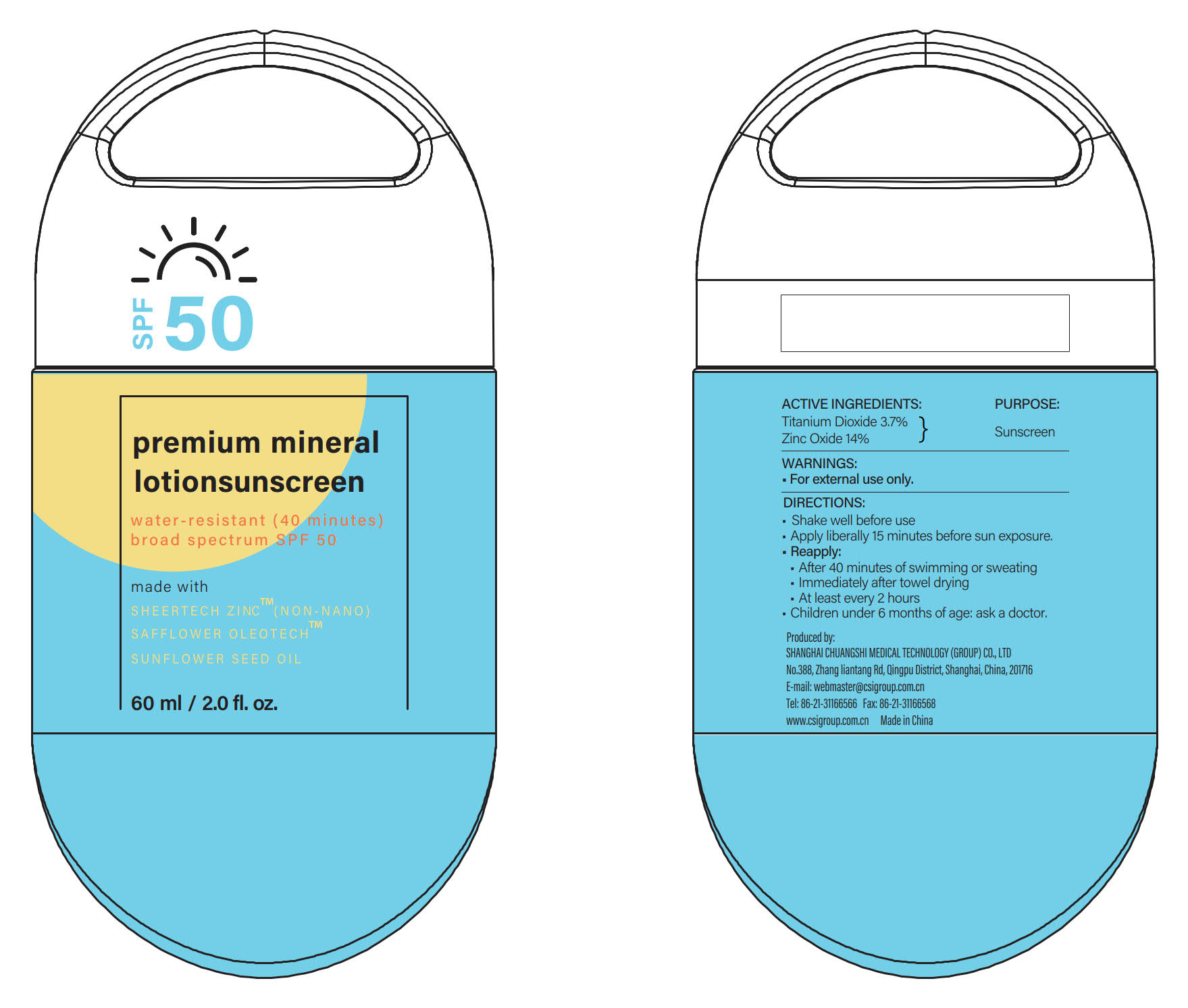
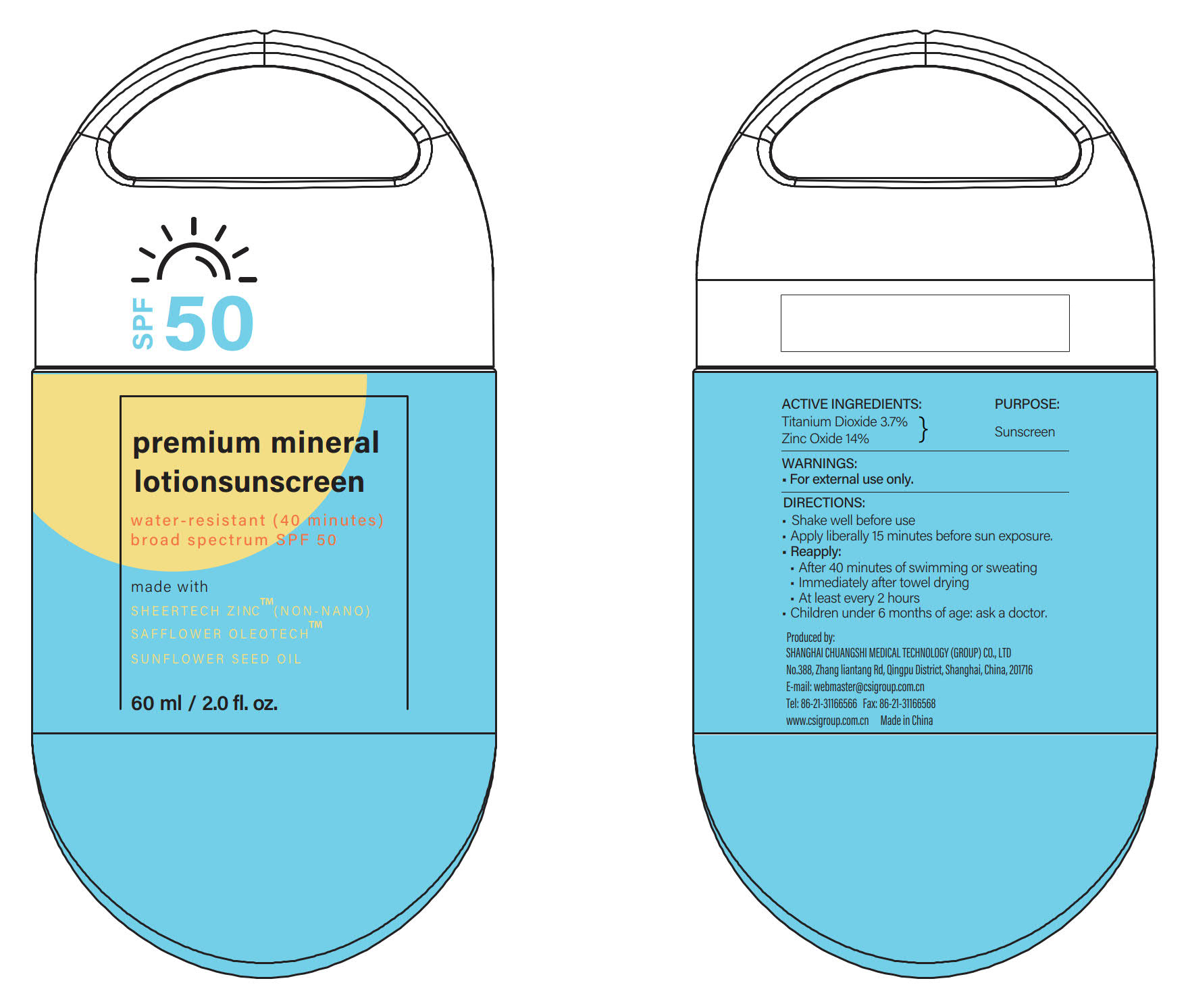
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- Do not use
- Stop use...
- When using this product
- ... Ask a doctor
- Keep out of reach of children
-
DIRECTIONS
- Shake well before use
- Apply liberally 15 minutes before sun exposure and as needed.
- Reapply:
·After 40 minutes of swimming or sweating
·Immediately after towel drying
·At least every 2 hours
- Children under 6 months of age: ask a doctor.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
·Limit your time in the sun, especially from 10 a.m. - 2 p.m.
·Wear long-sleeved shirts, pants, hats, and sunglasses.
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
Argania Spinosa Kernel Oil, Auminum Hydroxide, Water (Aqua), Bisbolol, Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Cucumis Sativus (Cucumber) Fruit Extract, Caesalpinia Spinosa Gum, Dicaprylyl Ether, C13-15 Alkane, Carthamus Tinctorius (Safflower) Oleosomes, Caprylyl Glycol, Cocos Nucifera (Coconut) Oil, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, Helianthus Annuus (Sunflower) Seed Oil, Hydroxyacetophenone, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Propanediol, Phenylpropanol, Pongamia Pinnata Seed Extract, Persea Gratissima (Avocado) Oil, Polyglyceryl-10 Mono/Dioleate, Polyhydroxystearic Acid, Rosa Rubiginosa Seed Oil, Stearyl Citrate, Sorbitan Sesquioleate, Silica, Stearic Acid, Sodium Phytate, Trioctyldodecyl Citrate, Tocopheryl Acetate, Vitis Vinifera (Grape) Seed Oil, 2-Hexanediol
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNSCREEN-LOTION
zinc oxide, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73557-575 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.037 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.14 g in 1 g Inactive Ingredients Ingredient Name Strength DICAPRYLYL ETHER (UNII: 77JZM5516Z) CARTHAMUS TINCTORIUS (SAFFLOWER) OLEOSOMES (UNII: 9S60Q72309) C13-15 ALKANE (UNII: 114P5I43UJ) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) PHYTATE SODIUM (UNII: 88496G1ERL) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) MONOSTEARYL CITRATE (UNII: YWW937R1QR) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TRIOCTYLDODECYL CITRATE (UNII: 35X8CT063R) PHENYLPROPANOL (UNII: 0F897O3O4M) SHEA BUTTER (UNII: K49155WL9Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) STEARIC ACID (UNII: 4ELV7Z65AP) LEVOMENOL (UNII: 24WE03BX2T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SUNFLOWER OIL (UNII: 3W1JG795YI) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) POLYGLYCERYL-10 DIOLEATE (UNII: 598RES7AXX) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) WATER (UNII: 059QF0KO0R) AVOCADO OIL (UNII: 6VNO72PFC1) CUCUMBER (UNII: YY7C30VXJT) CAESALPINIA SPINOSA RESIN (UNII: WL3883U2PO) ROSA RUBIGINOSA SEED OIL (UNII: 8M1P49MWAP) GRAPE SEED OIL (UNII: 930MLC8XGG) COCONUT OIL (UNII: Q9L0O73W7L) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ARGAN OIL (UNII: 4V59G5UW9X) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73557-575-03 67.5 g in 1 BOTTLE; Type 0: Not a Combination Product 09/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/13/2023 Labeler - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) Registrant - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) Establishment Name Address ID/FEI Business Operations Shanghai Chuangshi Medical Technology (Group) Co., Ltd. 546872672 label(73557-575) , pack(73557-575) Establishment Name Address ID/FEI Business Operations Kdc/One Chatsworth, Inc. 118542196 manufacture(73557-575)