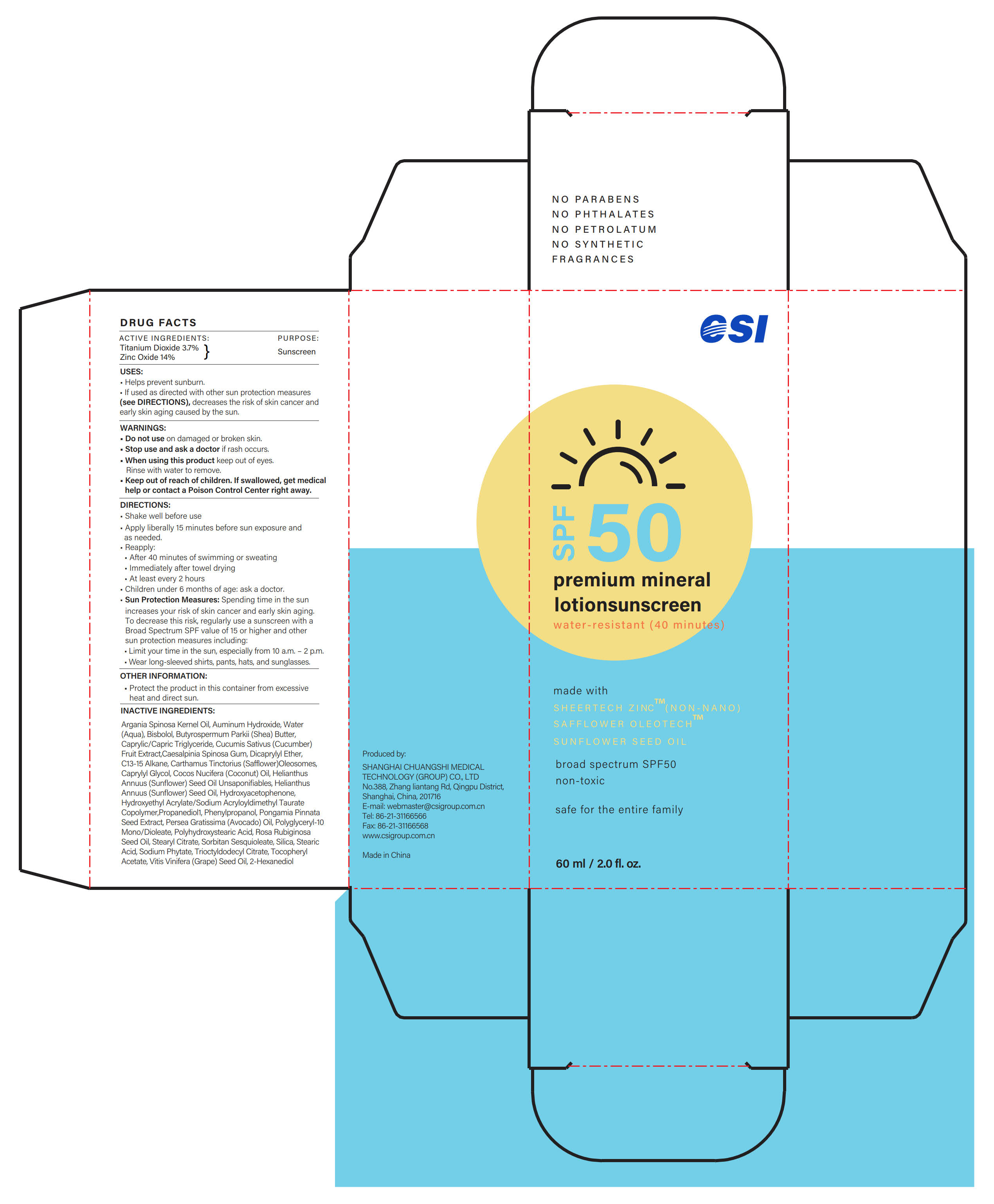
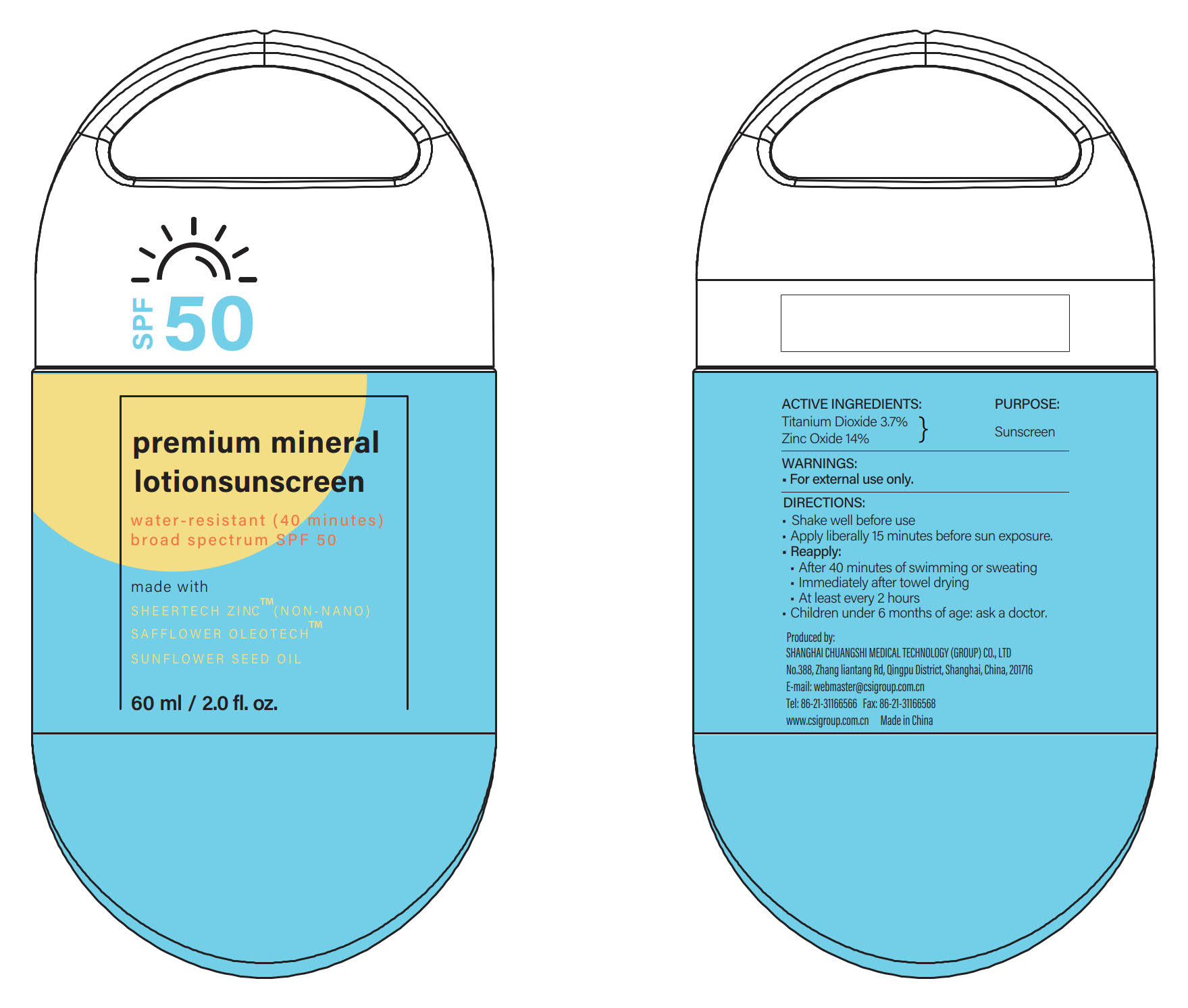
ACTIVE INGREDIENTS
Titanium Dioxide 3.7% ...... Purpose: Sunscreen
Zinc Oxide 14% ...... Purpose: Sunscreen
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures ( see DIRECTIONS), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- Shake well before use
- Apply liberally 15 minutes before sun exposure and as needed.
- Reapply:
·After 40 minutes of swimming or sweating
·Immediately after towel drying
·At least every 2 hours
- Children under 6 months of age: ask a doctor.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
·Limit your time in the sun, especially from 10 a.m. - 2 p.m.
·Wear long-sleeved shirts, pants, hats, and sunglasses.
INACTIVE INGREDIENTS
Argania Spinosa Kernel Oil, Auminum Hydroxide, Water (Aqua), Bisbolol, Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Cucumis Sativus (Cucumber) Fruit Extract, Caesalpinia Spinosa Gum, Dicaprylyl Ether, C13-15 Alkane, Carthamus Tinctorius (Safflower) Oleosomes, Caprylyl Glycol, Cocos Nucifera (Coconut) Oil, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, Helianthus Annuus (Sunflower) Seed Oil, Hydroxyacetophenone, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Propanediol, Phenylpropanol, Pongamia Pinnata Seed Extract, Persea Gratissima (Avocado) Oil, Polyglyceryl-10 Mono/Dioleate, Polyhydroxystearic Acid, Rosa Rubiginosa Seed Oil, Stearyl Citrate, Sorbitan Sesquioleate, Silica, Stearic Acid, Sodium Phytate, Trioctyldodecyl Citrate, Tocopheryl Acetate, Vitis Vinifera (Grape) Seed Oil, 2-Hexanediol