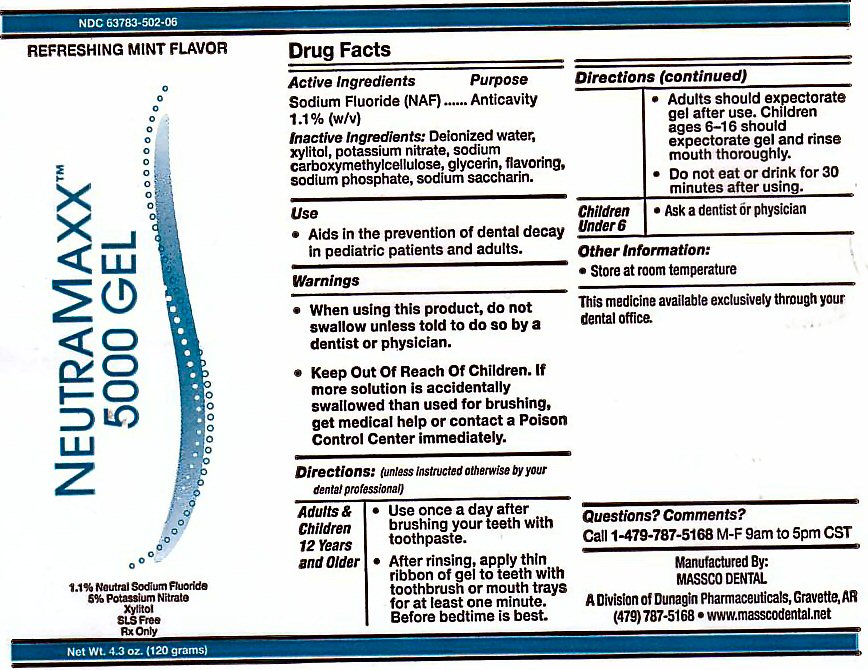
Label: NEUTRA MAXX 5000- sodium fluoride gel
- NDC Code(s): 63783-504-06
- Packager: Massco Dental A Division of Dunagin Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
DIRECTIONS: (UNLESS INSTRUCTED OTHERWISE BY YOUR DENTAL PROFESSIONAL)
ADULTS AND CHILDREN 6 YEARS AND OLDER: USE ONCE A DAY AFTER BRUSHING TEETH WITH TOOTHPASTE. AFTER RINSING, APPLY THIN RIBBON OF GEL TO TEETH WITH TOOTHBRUSH OR MOUTH TRAYS FOR AT LEASE ONE MINUTE. BEFORE BEDTIME IS BEST. ADULTS SHOULD EXPECTORATE AFTER USE. CHILDREN AGES 6-16 SHOULD EXPECTORATE GEL AND RINSE MOUTH THOROUGHLY. DO NOT EAT OR DRINK FOR 30 MINUTES AFTER USE. - WARNINGS
-
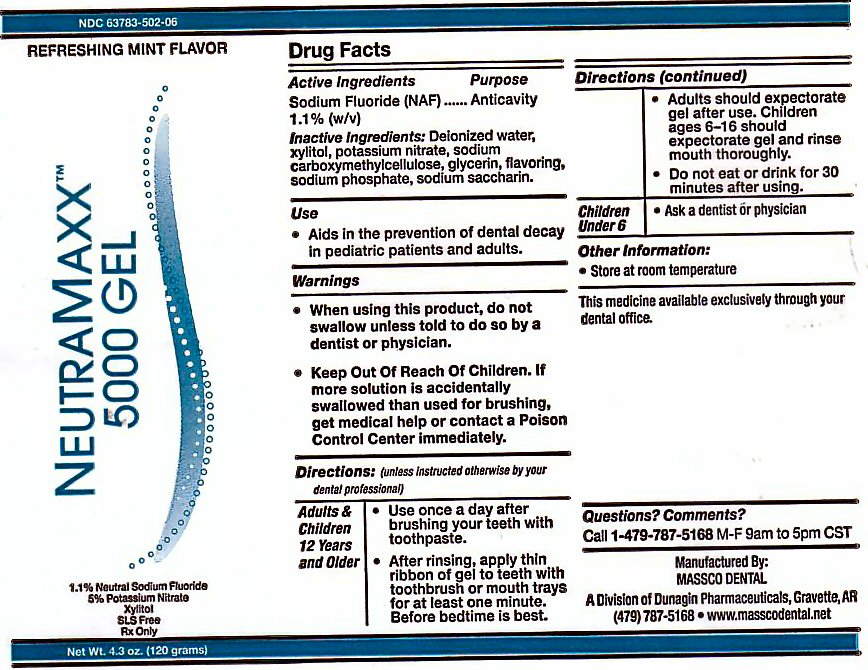
PACKAGE LABEL
NEUTRA MAXX 5000PPM GEL REFRESHING MINT FLAVOR
THE MAXIMUM AMOUNT OF FLUORIDE AVAILABLE 1.1% SODIUM FLUORIDE 5% POTASSIUM NITRATE XYLITOL SLS FREE Rx ONLY. NET WT 4.3 oz (120 g)
MANUFACTURED BY MASSCO DENTAL A DIVISION OF DUNAGIN PHARMACEUTICALS GRAVETTE, AR 72736 THE MEDICINE AVAILABLE EXCLUSIVELY THROUGH YOUR DENTAL OFFICE.
OTHER INFORMATION: STORE AT ROOM TEMPERATURE QUESTION? COMMENTS? CALL 1-479-787-5168 M-F 9AM TO 5PM CST
-
INGREDIENTS AND APPEARANCE
NEUTRA MAXX 5000
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1.428 g in 120 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) POTASSIUM NITRATE (UNII: RU45X2JN0Z) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) SODIUM PHOSPHATE (UNII: SE337SVY37) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor MINT (Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-504-06 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 Labeler - Massco Dental A Division of Dunagin Pharmaceuticals (008081858) Registrant - Massco Dental A Division of Dunagin Pharmaceuticals (008081858) Establishment Name Address ID/FEI Business Operations Massco Dental A Division of Dunagin Pharmaceuticals 008081858 manufacture(63783-504)