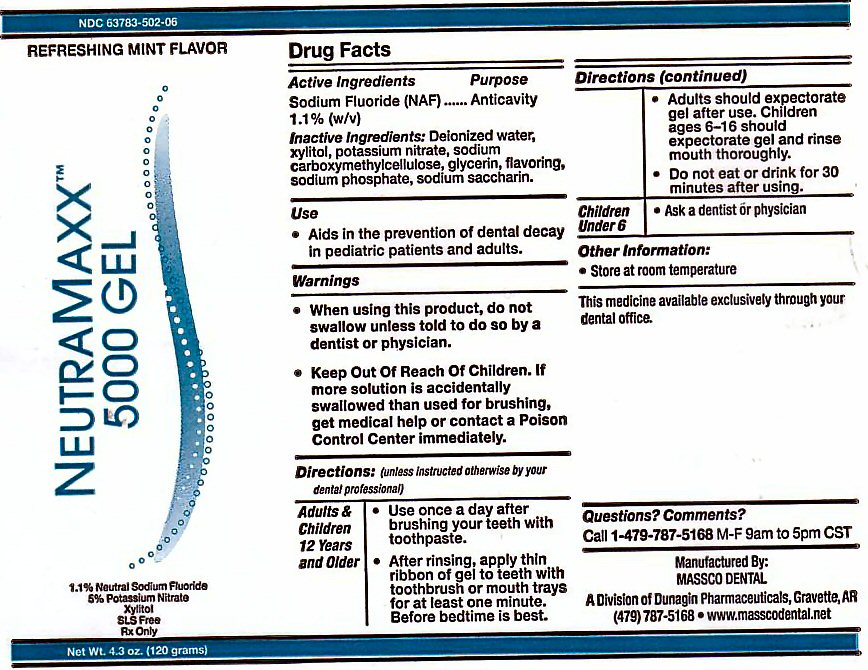
ACTIVE INGREDIENTS
ACTIVE INGREDIENT PURPOSE
SODIUM FLUORIDE (NaF) 1.1% (w/v) ANTICAVITY
INACTIVE INGREDIENTS
DEIONIZED WATER, XYLITOL, POTASSIUM NITRATE, SODIUM CARBOXYMETHYLCELLOSE, GLYCERIN, FLAVORING,SODIUM SACCHARIN, SODIUM PHOSPHATE .
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN. IF MORE SOLUTION IS ACCIDENTALLY SWALLOWED THAN USED FOR BRUSHING, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
DIRECTIONS
DIRECTIONS: (UNLESS INSTRUCTED OTHERWISE BY YOUR DENTAL PROFESSIONAL)
ADULTS AND CHILDREN 6 YEARS AND OLDER: USE ONCE A DAY AFTER BRUSHING TEETH WITH TOOTHPASTE. AFTER RINSING, APPLY THIN RIBBON OF GEL TO TEETH WITH TOOTHBRUSH OR MOUTH TRAYS FOR AT LEASE ONE MINUTE. BEFORE BEDTIME IS BEST. ADULTS SHOULD EXPECTORATE AFTER USE. CHILDREN AGES 6-16 SHOULD EXPECTORATE GEL AND RINSE MOUTH THOROUGHLY. DO NOT EAT OR DRINK FOR 30 MINUTES AFTER USE.
PACKAGE LABEL
NEUTRA MAXX 5000PPM GEL REFRESHING MINT FLAVOR
THE MAXIMUM AMOUNT OF FLUORIDE AVAILABLE 1.1% SODIUM FLUORIDE 5% POTASSIUM NITRATE XYLITOL SLS FREE Rx ONLY. NET WT 4.3 oz (120 g)
MANUFACTURED BY MASSCO DENTAL A DIVISION OF DUNAGIN PHARMACEUTICALS GRAVETTE, AR 72736 THE MEDICINE AVAILABLE EXCLUSIVELY THROUGH YOUR DENTAL OFFICE.
OTHER INFORMATION: STORE AT ROOM TEMPERATURE QUESTION? COMMENTS? CALL 1-479-787-5168 M-F 9AM TO 5PM CST