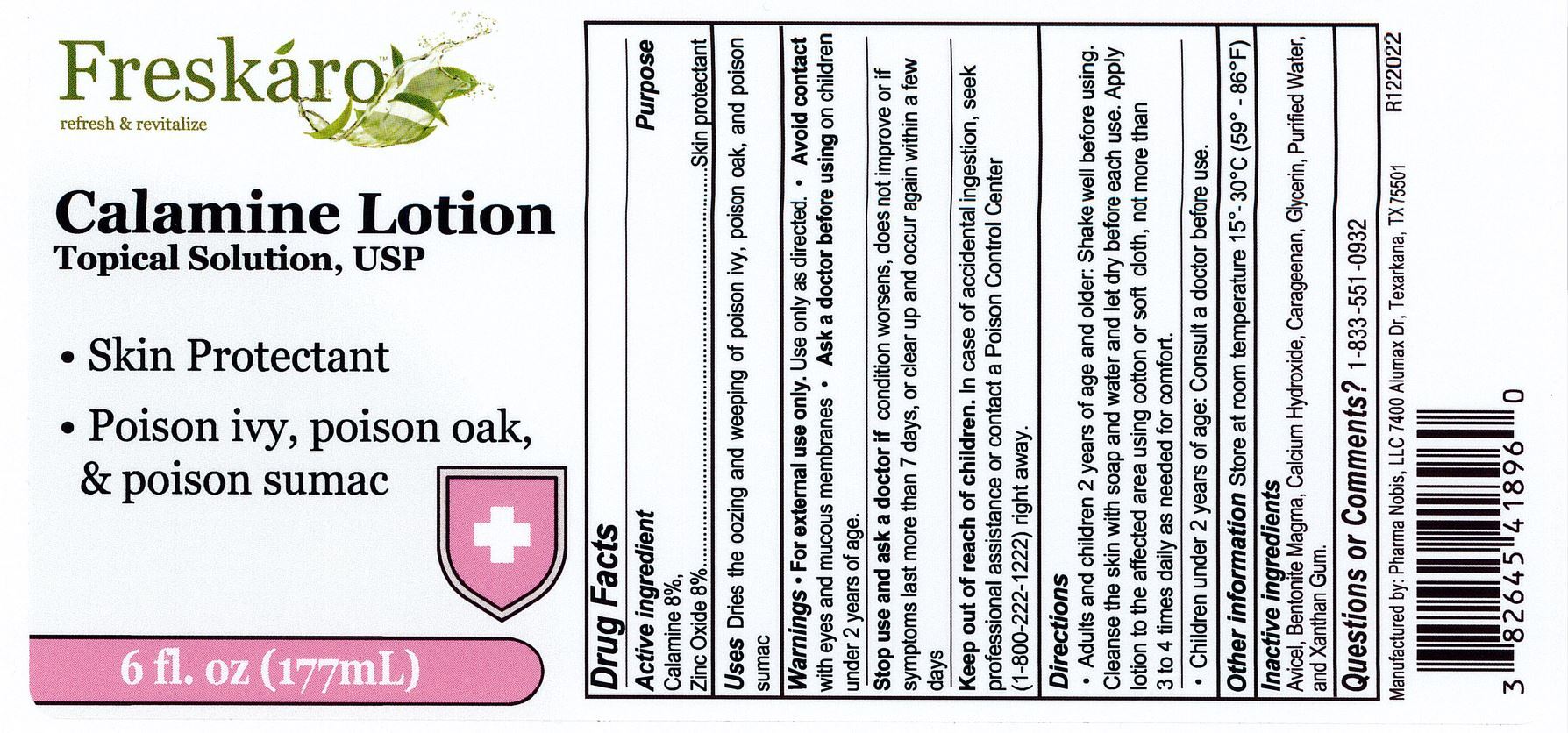
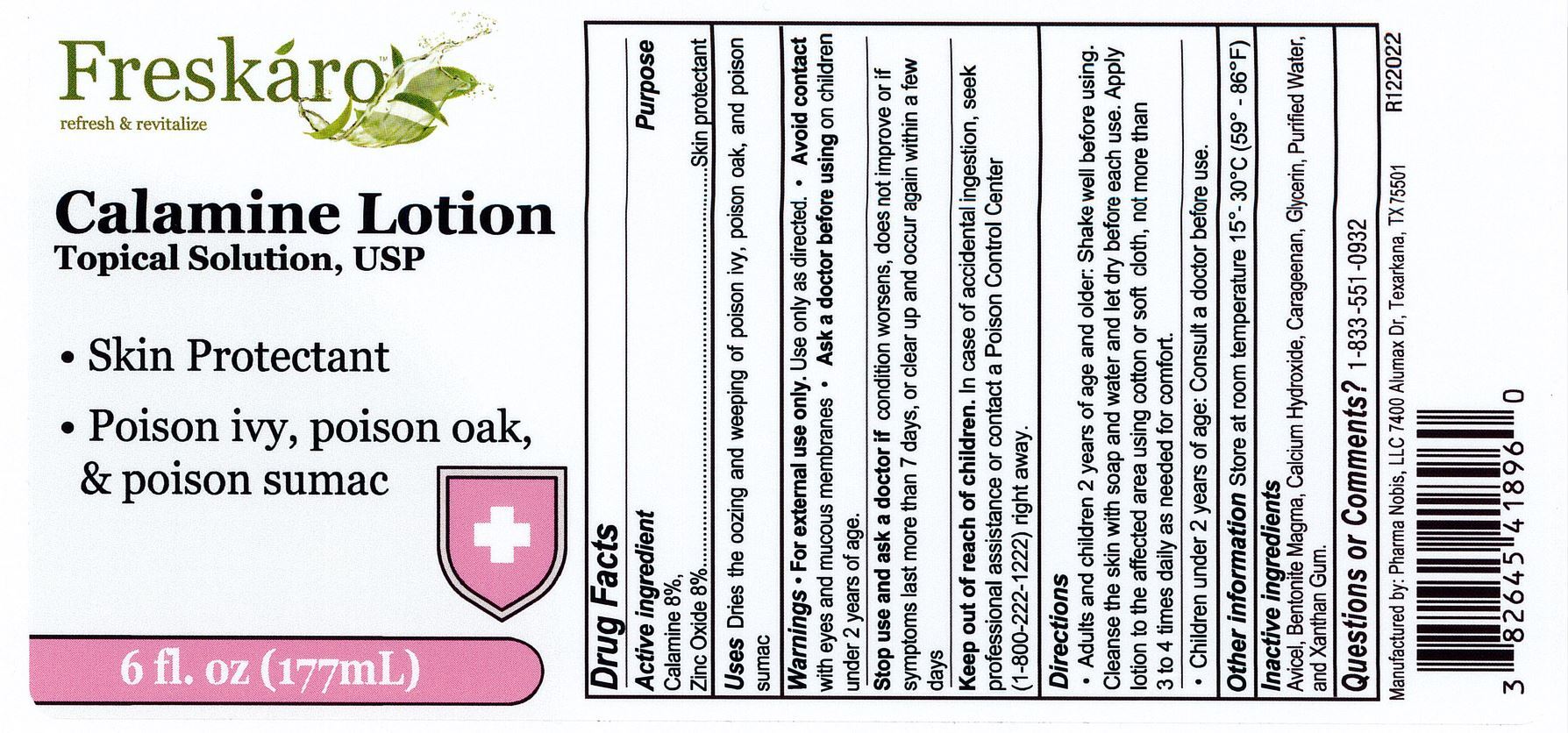
Label: CALAMINE- calamine and zinc oxide lotion
- NDC Code(s): 82645-418-96
- Packager: Pharma Nobis, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Uses
- Purpose
- Warnings
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
Adults and chidren 2 years of age and older: shake well before using. Cleanse the skin with soap and water and let it dry befroe each use. Apply lotion to the affected area using a cotton or soft cloth, not more than 3 to 4 times daily as needed for comfort.
Children under 2 years of age: Consult a doctor before use
- Other information
- Inactive ingredients
- Questions
- label
-
INGREDIENTS AND APPEARANCE
CALAMINE
calamine and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82645-418 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 80 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENTONITE (UNII: A3N5ZCN45C) CARRAGEENAN (UNII: 5C69YCD2YJ) XANTHAN GUM (UNII: TTV12P4NEE) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82645-418-96 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/16/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/16/2023 Labeler - Pharma Nobis, LLC (118564114) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 label(82645-418) , manufacture(82645-418) , analysis(82645-418) , pack(82645-418)