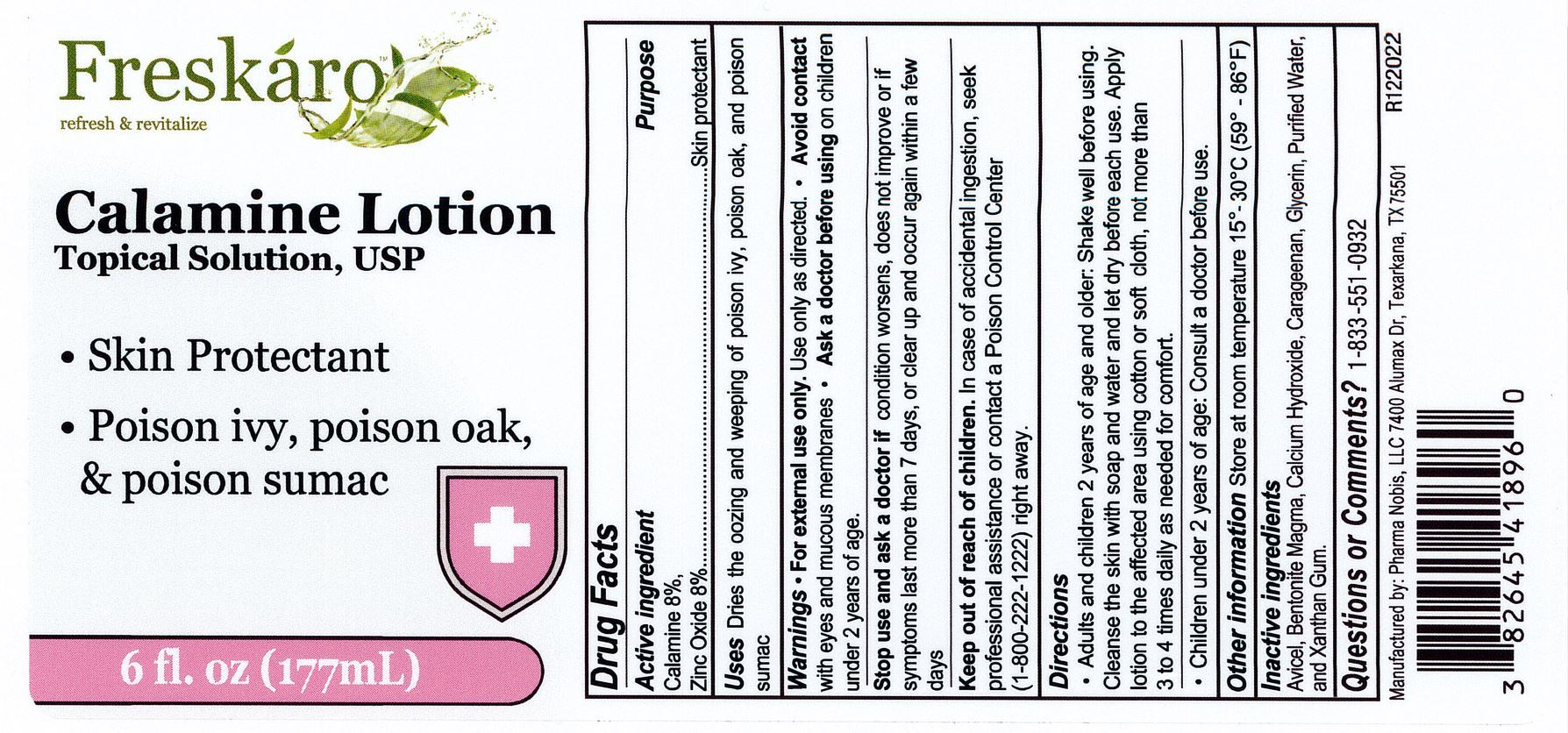
Warnings
For external use only. Use only as directed.
Avoid contact with eyes and mucous membranes.
Ask a doctor before using on chilren under 2 years of age.
Stop use and ask a doctor if
condition worsens, does not improve or if symptoms last more than 7 days, or clear up and occur again within a few days.
Keep out of reach of children
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Adults and chidren 2 years of age and older: shake well before using. Cleanse the skin with soap and water and let it dry befroe each use. Apply lotion to the affected area using a cotton or soft cloth, not more than 3 to 4 times daily as needed for comfort.
Children under 2 years of age: Consult a doctor before use