Label: OXYBUTYNIN CHLORIDE tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5743-0, 54868-5743-1, 54868-5743-2, 54868-5743-3 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0378-6015
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 21, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Oxybutynin chloride is an antispasmodic, anticholinergic agent. Each Oxybutynin Chloride Extended Release Tablet contains 15 mg of oxybutynin chloride USP, formulated as a once-a-day controlled-release tablet for oral administration. Oxybutynin chloride is administered as a racemate of R- and S-enantiomers.
Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochloride. The empirical formula of oxybutynin chloride is C 22 H 31 NO 3 •HCl.
Its structural formula is:
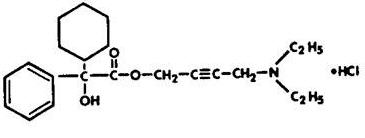
Oxybutynin chloride is a white crystalline solid with a molecular weight of 393.9. It is readily soluble in water and acids, but relatively insoluble in alkalis.
Oxybutynin Chloride Extended Release Tablets also contain the following inert ingredients: cellulose acetate, hypromellose, lactose, magnesium stearate, polyethylene glycol, polyethylene oxide, synthetic iron oxides, titanium dioxide, polysorbate 80, sodium chloride, and butylated hydroxytoluene.
System Components and PerformanceOxybutynin Chloride Extended Release Tablets use osmotic pressure to deliver oxybutynin chloride at a controlled rate over approximately 24 hours. The system, which resembles a conventional tablet in appearance, comprises an osmotically active bilayer core surrounded by a semipermeable membrane. The bilayer core is composed of a drug layer containing the drug and excipients, and a push layer containing osmotically active components. There is a precision-laser drilled orifice in the semipermeable membrane on the drug-layer side of the tablet. In an aqueous environment, such as the gastrointestinal tract, water permeates through the membrane into the tablet core, causing the drug to go into suspension and the push layer to expand. This expansion pushes the suspended drug out through the orifice. The semipermeable membrane controls the rate at which water permeates into the tablet core, which in turn controls the rate of drug delivery. The controlled rate of drug delivery into the gastrointestinal lumen is thus independent of pH or gastrointestinal motility. The function of Oxybutynin Chloride Extended Release Tablets depends on the existence of an osmotic gradient between the contents of the bilayer core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
-
CLINICAL PHARMACOLOGY
Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. Oxybutynin chloride exhibits only one-fifth of the anticholinergic activity of atropine on the rabbit detrusor muscle, but four to ten times the antispasmodic activity. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Oxybutynin chloride relaxes bladder smooth muscle. In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void. Oxybutynin thus decreases urgency and the frequency of both incontinent episodes and voluntary urination.
Antimuscarinic activity resides predominantly in the R-isomer. A metabolite, desethyloxybutynin, has pharmacological activity similar to that of oxybutynin in in vitro studies.
PharmacokineticsAbsorptionFollowing the first dose of Oxybutynin Chloride Extended Release Tablets, oxybutynin plasma concentrations rise for 4 to 6 hours; thereafter steady concentrations are maintained for up to 24 hours, minimizing fluctuations between peak and trough concentrations associated with oxybutynin.
The relative bioavailabilities of R- and S-oxybutynin from Oxybutynin Chloride Extended Release Tablets are 156% and 187%, respectively, compared with oxybutynin. The mean pharmacokinetic parameters for R- and S-oxybutynin are summarized in Table 1. The plasma concentration-time profiles for R- and S-oxybutynin are similar in shape; Figure 1 shows the profile for R-oxybutynin.
Table 1 Mean (SD) R- and S-Oxybutynin Pharmacokinetic Parameters Following a Single Dose of Oxybutynin Chloride Extended Release Tablets 10 mg (n=43) Parameters (units)
R-Oxybutynin
S-Oxybutynin
C max (ng/mL) 1.0
(0.6)
1.8
(1.0)
T max (h) 12.7
(5.4)
11.8
(5.3)
t 1/2 (h) 13.2
(6.2)
12.4
(6.1)
AUC (0–48) (ng∙h/mL)
18.4
(10.3)
34.2
(16.9)
AUC inf (ng∙h/mL) 21.3
(12.2)
39.5
(21.2)
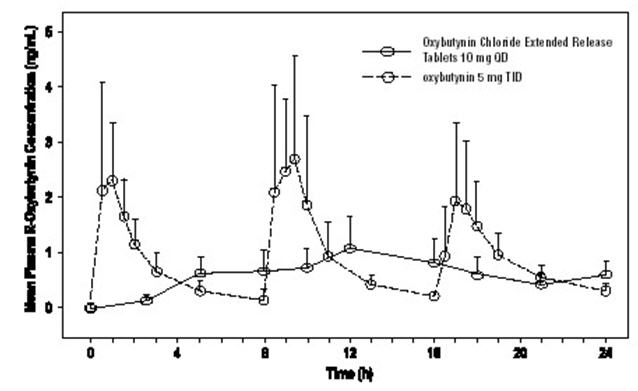
Figure 1. Mean R-oxybutynin plasma concentrations following a single dose of Oxybutynin Chloride Extended Release Tablets 10 mg and oxybutynin 5 mg administered every 8 hours (n=23 for each treatment).Steady state oxybutynin plasma concentrations are achieved by Day 3 of repeated Oxybutynin Chloride Extended Release Tablets dosing, with no observed drug accumulation or change in oxybutynin and desethyloxybutynin pharmacokinetic parameters.
Oxybutynin Chloride Extended Release Tablets steady state pharmacokinetics was studied in 19 children aged 5–15 years with detrusor overactivity associated with a neurological condition (e.g. spina bifida). The children were on Oxybutynin Chloride Extended Release Tablets total daily dose ranging from 5 to 20 mg (0.10 to 0.77 mg/kg). Sparse sampling technique was used to obtain serum samples. When all available data are normalized to an equivalent of 5 mg per day Oxybutynin Chloride Extended Release Tablets, the mean pharmacokinetic parameters derived for R- and S-oxybutynin and R- and S-desethyloxybutynin are summarized in Table 2. The plasma-time concentration profiles for R- and S-oxybutynin are similar in shape; Figure 2 shows the profile for R-oxybutynin when all available data are normalized to an equivalent of 5 mg per day.
Table 2 Mean ± SD R- and S-Oxybutynin and R- and S-Desethyloxybutynin Pharmacokinetic Parameters in Children Aged 5–15 Following Administration of 5 to 20 mg Oxybutynin Chloride Extended Release Tablets Once Daily (n=19) All Available Data Normalized to an Equivalent of Oxybutynin Chloride Extended Release Tablets 5 mg Once Daily
R-Oxybutynin
S-Oxybutynin
R- Desethyloxybutynin
S- Desethyloxybutynin
C max (ng/mL)
0.7 ± 0.4 1.3 ± 0.8 7.8 ± 3.7 4.2 ± 2.3 T max (hr) 5.0
5.0
5.0
5.0
AUC (ng∙hr/mL) 12.8 ± 7.0 23.7 ± 14.4 125.1 ± 66.7 73.6 ± 47.7 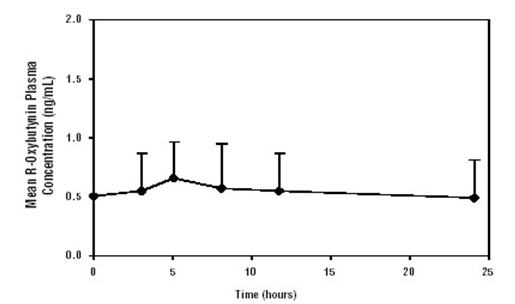
Figure 2. Mean steady state (±SD) R-oxybutynin plasma concentrations following administration of 5 to 20 mg Oxybutynin Chloride Extended Release Tablets once daily in children aged 5–15. Plot represents all available data normalized to an equivalent of Oxybutynin Chloride Extended Release Tablets 5 mg once daily.
Food EffectsThe rate and extent of absorption and metabolism of oxybutynin are similar under fed and fasted conditions.
DistributionPlasma concentrations of oxybutynin decline biexponentially following intravenous or oral administration. The volume of distribution is 193 L after intravenous administration of 5 mg oxybutynin chloride.
MetabolismOxybutynin is metabolized primarily by the cytochrome P450 enzyme systems, particularly CYP3A4 found mostly in the liver and gut wall. Its metabolic products include phenylcyclohexylglycolic acid, which is pharmacologically inactive, and desethyloxybutynin, which is pharmacologically active. Following Oxybutynin Chloride Extended Release Tablet administration, plasma concentrations of R- and S-desethyloxybutynin are 73% and 92%, respectively, of concentrations observed with oxybutynin.
ExcretionOxybutynin is extensively metabolized by the liver, with less than 0.1% of the administered dose excreted unchanged in the urine. Also, less than 0.1% of the administered dose is excreted as the metabolite desethyloxybutynin.
Dose ProportionalityPharmacokinetic parameters of oxybutynin and desethyloxybutynin (C max and AUC) following administration of 5–20 mg of Oxybutynin Chloride Extended Release Tablets are dose proportional.
Special PopulationsGeriatricThe pharmacokinetics of Oxybutynin Chloride Extended Release Tablets were similar in all patients studied (up to 78 years of age).
PediatricThe pharmacokinetics of Oxybutynin Chloride Extended Release Tablets were evaluated in 19 children aged 5–15 years with detrusor overactivity associated with a neurological condition (e.g., spina bifida). The pharmacokinetics of Oxybutynin Chloride Extended Release Tablets in these pediatric patients were consistent with those reported for adults (see Tables 1 and 2 , and Figures 1 and 2 above).
GenderThere are no significant differences in the pharmacokinetics of oxybutynin in healthy male and female volunteers following administration of Oxybutynin Chloride Extended Release Tablets.
RaceAvailable data suggest that there are no significant differences in the pharmacokinetics of oxybutynin based on race in healthy volunteers following administration of Oxybutynin Chloride Extended Release Tablets.
Renal InsufficiencyThere is no experience with the use of Oxybutynin Chloride Extended Release Tablets in patients with renal insufficiency.
Hepatic InsufficiencyThere is no experience with the use of Oxybutynin Chloride Extended Release Tablets in patients with hepatic insufficiency.
Drug-Drug InteractionsSee PRECAUTIONS: Drug Interactions .
-
CLINICAL STUDIES
Oxybutynin Chloride Extended Release Tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in three controlled studies and one open label study. The majority of patients were Caucasian (89.0%) and female (91.9%) with a mean age of 59 years (range, 18 to 98 years). Entry criteria required that patients have urge or mixed incontinence (with a predominance of urge) as evidenced by ≥ 6 urge incontinence episodes per week and ≥ 10 micturitions per day. Study 1 was a fixed dose escalation design, whereas the other studies used a dose adjustment design in which each patient's final dose was adjusted to a balance between improvement of incontinence symptoms and tolerability of side effects. Controlled studies included patients known to be responsive to oxybutynin or other anticholinergic medications, and these patients were maintained on a final dose for up to 2 weeks.
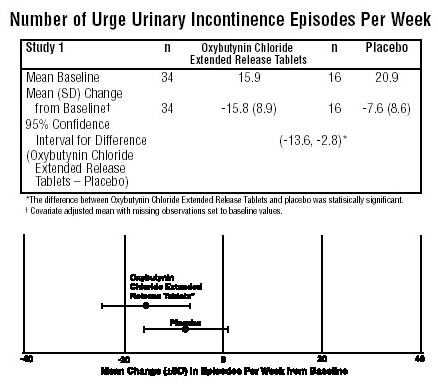
The efficacy results for the three controlled trials are presented in the following tables and figures.
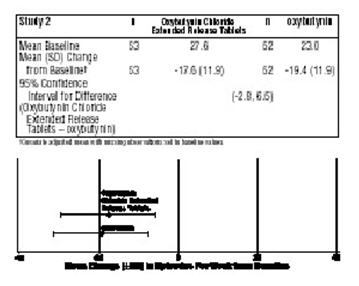

-
INDICATIONS AND USAGE
Oxybutynin Chloride Extended Release Tablets are a once-daily controlled-release tablet indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
Oxybutynin Chloride Extended Release Tablets are also indicated in the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida).
-
CONTRAINDICATIONS
Oxybutynin Chloride Extended Release Tablets are contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled narrow-angle glaucoma and in patients who are at risk for these conditions.
Oxybutynin Chloride Extended Release Tablets are also contraindicated in patients who have demonstrated hypersensitivity to the drug substance or other components of the product.
-
PRECAUTIONS
Central Nervous System Effects
Oxybutynin is associated with anticholinergic central nervous system (CNS) effects (See ADVERSE REACTIONS ). A variety of CNS anticholinergic effects have been reported, including hallucinations, agitation, confusion and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly in the first few months after beginning treatment or increasing the dose. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
Oxybutynin Chloride Extended Release Tablets should be used with caution in patients with preexisting dementia treated with cholinesterase inhibitors due to the risk of aggravation of symptoms.
GeneralOxybutynin Chloride Extended Release Tablets should be used with caution in patients with hepatic or renal impairment and in patients with myasthenia gravis due to the risk of symptom aggravation.
Urinary RetentionOxybutynin Chloride Extended Release Tablets should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention (see CONTRAINDICATIONS ).
Gastrointestinal DisordersOxybutynin Chloride Extended Release Tablets should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention (see CONTRAINDICATIONS ).
Oxybutynin Chloride Extended Release Tablets, like other anticholinergic drugs, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis and intestinal atony.
Oxybutynin Chloride Extended Release Tablets should be used with caution in patients who have gastroesophageal reflux and/or who are concurrently taking drugs (such as bisphosphonates) that can cause or exacerbate esophagitis.
As with any other nondeformable material, caution should be used when administering Oxybutynin Chloride Extended Release Tablets to patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic). There have been rare reports of obstructive symptoms in patients with known strictures in association with the ingestion of other drugs in nondeformable controlled-release formulations.
Information for PatientsPatients should be informed that heat prostration (fever and heat stroke due to decreased sweating) can occur when anticholinergics such as oxybutynin chloride are administered in the presence of high environmental temperature.
Because anticholinergic agents such as oxybutynin may produce drowsiness (somnolence) or blurred vision, patients should be advised to exercise caution.
Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents such as oxybutynin.
Patients should be informed that Oxybutynin Chloride Extended Release Tablets should be swallowed whole with the aid of liquids. Patients should not chew, divide, or crush tablets. The medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell is eliminated from the body; patients should not be concerned if they occasionally notice in their stool something that looks like a tablet.
Oxybutynin Chloride Extended Release Tablets should be taken at approximately the same time each day.
Drug InteractionsThe concomitant use of oxybutynin with other anticholinergic drugs or with other agents which produce dry mouth, constipation, somnolence (drowsiness), and/or other anticholinergic-like effects may increase the frequency and/or severity of such effects.
Anticholinergic agents may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility. This may be of concern for drugs with a narrow therapeutic index.
Mean oxybutynin chloride plasma concentrations were approximately 2 fold higher when Oxybutynin Chloride Extended Release Tablets were administered with ketoconazole, a potent CYP3A4 inhibitor. Other inhibitors of the cytochrome P450 3A4 enzyme system, such as antimycotic agents (e.g., itraconazole and miconazole) or macrolide antibiotics (e.g., erythromycin and clarithromycin), may alter oxybutynin mean pharmacokinetic parameters (i.e., C max and AUC). The clinical relevance of such potential interactions is not known. Caution should be used when such drugs are co-administered.
Carcinogenesis, Mutagenesis, Impairment of FertilityA 24-month study in rats at dosages of oxybutynin chloride of 20, 80, and 160 mg/kg/day showed no evidence of carcinogenicity. These doses are approximately 6, 25, and 50 times the maximum human exposure, based on surface area.
Oxybutynin chloride showed no increase of mutagenic activity when tested in Schizosaccharomyces pompholiciformis, Saccharomyces cerevisiae, and Salmonella typhimurium test systems.
Reproduction studies with oxybutynin chloride in the mouse, rat, hamster, and rabbit showed no definite evidence of impaired fertility.
PregnancyTeratogenic EffectsPregnancy Category BReproduction studies with oxybutynin chloride in the mouse, rat, hamster, and rabbit showed no definite evidence of impaired fertility or harm to the animal fetus. The safety of Oxybutynin Chloride Extended Release Tablet administration to women who are or who may become pregnant has not been established. Therefore, Oxybutynin Chloride Extended Release Tablets should not be given to pregnant women unless, in the judgment of the physician, the probable clinical benefits outweigh the possible hazards.
Nursing MothersIt is not known whether oxybutynin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Oxybutynin Chloride Extended Release Tablets are administered to a nursing woman.
Pediatric UseThe safety and efficacy of Oxybutynin Chloride Extended Release Tablets were studied in 60 children in a 24-week, open-label trial. Patients were aged 6–15 years, all had symptoms of detrusor overactivity in association with a neurological condition (e.g., spina bifida), all used clean intermittent catheterization, and all were current users of oxybutynin chloride. Study results demonstrated that administration of Oxybutynin Chloride Extended Release Tablets 5 to 20 mg/day was associated with an increase from baseline in mean urine volume per catheterization from 108 mL to 136 mL, an increase from baseline in mean urine volume after morning awakening from 148 mL to 189 mL, and an increase from baseline in the mean percentage of catheterizations without a leaking episode from 34% to 51%.
Urodynamic results were consistent with clinical results. Administration of Oxybutynin Chloride Extended Release Tablets resulted in an increase from baseline in mean maximum cystometric capacity from 185 mL to 254 mL, a decrease from baseline in mean detrusor pressure at maximum cystometric capacity from 44 cm H 2 O to 33 cm H 2 O, and a reduction in the percentage of patients demonstrating uninhibited detrusor contractions (of at least 15 cm H 2 O) from 60% to 28%.
Oxybutynin Chloride Extended Release Tablets are not recommended in pediatric patients who can not swallow the tablet whole without chewing, dividing, or crushing, or in children under the age of 6 (See DOSAGE AND ADMINISTRATION ).
Geriatric UseThe rate and severity of anticholinergic effects reported by patients less than 65 years old and those 65 years and older were similar (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations: Geriatric ).
-
ADVERSE REACTIONS
Adverse Events with Oxybutynin Chloride Extended Release Tablets
The safety and efficacy of Oxybutynin Chloride Extended Release Tablets was evaluated in a total of 580 participants who received Oxybutynin Chloride Extended Release Tablets in 4 clinical trials (429 patients) and four pharmacokinetic studies (151 healthy volunteers). The 429 patients were treated with 5–30 mg/day for up to 4.5 months. Three of the 4 clinical trials allowed dose adjustments based on efficacy and adverse events and one was a fixed dose escalation design. Safety information is provided for 429 patients from these three controlled clinical studies and one open label study in the first column of Table 3 below.
Adverse events from two additional fixed dose, active controlled, 12 week treatment duration, postmarketing studies, in which 576 patients were treated with Oxybutynin Chloride Extended Release Tablets 10 mg/day, are also listed in Table 3 (second column). The adverse events are reported regardless of causality.
Table 3 Incidence (%) of Adverse Events Reported by ≥ 5% of Patients Using Oxybutynin Chloride Extended Release Tablets (5–30 mg/day) and % of Corresponding Adverse Events in Two Fixed Dose (10mg/day) Studies
Oxybutynin Chloride Extended
Release Tablets
Oxybutynin Chloride Extended
Release Tablets
Body System
Adverse Event
5-30 mg/day
(n=429)
10 mg/day
(n=576)
General
headache
10
6
asthenia
7
3
pain
7
4
Digestive
dry mouth
61
29
constipation
13
7
diarrhea
9
7
nausea
9
2
dyspepsia
7
5
Nervous
somnolence
12
2
dizziness
6
4
Respiratory
rhinitis
6
2
Special senses
blurred vision
8
1
dry eyes
6
3
Urogenital
urinary tract infection
5
5
The most common adverse events reported by the 429 patients receiving 5–30 mg/day Oxybutynin Chloride Extended Release Tablets were the expected side effects of anticholinergic agents. The incidence of dry mouth was dose-related.
The discontinuation rate for all adverse events was 6.8% in the 429 patients from the 4 studies of efficacy and safety who received 5–30 mg/day. The most frequent adverse event causing early discontinuation of study medication was nausea (1.9%), while discontinuation due to dry mouth was 1.2%.
In addition, the following adverse events were reported by greater than or equal to 1 to less than 5% of all patients who received Oxybutynin Chloride Extended Release Tablets in the 6 adjustable and fixed dose efficacy and safety studies. Infections and infestations: nasopharyngitis, upper respiratory tract infection, sinusitis, bronchitis, cystitis; Psychiatric disorders: insomnia, depression, nervousness, confusional state; Nervous System Disorders: dysgeusia; Cardiac disorders: palpitations; Vascular disorders: hypertension; Respiratory, thoracic and mediastinal disorders: nasal dryness, cough, pharyngolaryngeal pain, dry throat; Gastrointestinal Disorders: gastroesophageal reflux disease, abdominal pain, loose stools, flatulence, vomiting; Skin and subcutaneous tissue disorders: dry skin, pruritis; Musculoskeletal and connective tissue disorders: back pain, arthralgia, pain in extremity; Renal and urinary disorders: urinary retention, urinary hesitation, dysuria; General disorders and administration site conditions: fatigue, edema peripheral, asthenia, chest pain; Investigations: blood pressure increased.
Postmarketing SurveillanceBecause postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following additional adverse drug reactions have been reported from worldwide postmarketing experience with Oxybutynin Chloride Extended Release Tablets: Psychiatric Disorders: psychotic disorder, agitation, hallucinations, memory impairment; Nervous System Disorders: convulsions; Cardiac Disorders: arrhythmia; tachycardia, QT interval prolongation; Vascular Disorders: flushing; Skin and Subcutaneous Tissue Disorders: rash; Renal and Urinary Disorders: impotence; Injury, poisoning and procedural complications: fall.
Additional adverse events reported with some other oxybutynin chloride formulations include: cycloplegia, mydriasis, and suppression of lactation.
-
OVERDOSAGE
The continuous release of oxybutynin from Oxybutynin Chloride Extended Release Tablets should be considered in the treatment of overdosage. Patients should be monitored for at least 24 hours. Treatment should be symptomatic and supportive. Activated charcoal as well as a cathartic may be administered.
Overdosage with oxybutynin chloride has been associated with anticholinergic effects including central nervous system excitation, flushing, fever, dehydration, cardiac arrhythmia, vomiting, and urinary retention.
Ingestion of 100 mg oxybutynin chloride in association with alcohol has been reported in a 13-year-old boy who experienced memory loss, and a 34-year-old woman who developed stupor, followed by disorientation and agitation on awakening, dilated pupils, dry skin, cardiac arrhythmia, and retention of urine. Both patients fully recovered with symptomatic treatment.
-
DOSAGE AND ADMINISTRATION
Oxybutynin Chloride Extended Release Tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed.
Oxybutynin Chloride Extended Release Tablets may be administered with or without food.
AdultsThe recommended starting dose of Oxybutynin Chloride Extended Release Tablets is 5 or 10 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 30 mg/day). In general, dosage adjustment may proceed at approximately weekly intervals.
Pediatric Patients Aged 6 Years of Age and OlderThe recommended starting dose of Oxybutynin Chloride Extended Release Tablets is 5 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 20 mg/day).
-
HOW SUPPLIED
Oxybutynin Chloride Extended Release Tablets are available containing 15 mg of oxybutynin chloride, USP. The 15 mg round, gray, tablets are imprinted on one side with M over O 17. The 15 mg Oxybutynin Chloride Extended Release Tablets are supplied in:
bottles of 10 tablets NDC 54868-5743-1
Storage
bottles of 30 tablets NDC 54868-5743-0
bottles of 90 tablets NDC 54868-5743-2
bottles of 180 tablets NDC 54868-5743-3
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from moisture and humidity.
Manufactured by
ALZA Corporation, Vacaville, CA 95688Placeholder for ALZA Corporation Logo
An ALZA OROS ®
Technology ProductOROS ® is a registered trademark of ALZA Corporation.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505Placeholder for Mylan Pharmaceuticals Inc. Logo
10151902
Revised July 2009
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OXYBUTYNIN CHLORIDE
oxybutynin chloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5743(NDC:0378-6015) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYBUTYNIN CHLORIDE (UNII: L9F3D9RENQ) (OXYBUTYNIN - UNII:K9P6MC7092) OXYBUTYNIN CHLORIDE 15 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Product Characteristics Color gray Score no score Shape ROUND Size 8mm Flavor Imprint Code M;O;17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5743-0 30 in 1 BOTTLE 2 NDC:54868-5743-1 10 in 1 BOTTLE 3 NDC:54868-5743-2 90 in 1 BOTTLE 4 NDC:54868-5743-3 180 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078293 01/13/2010 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 repack, relabel


