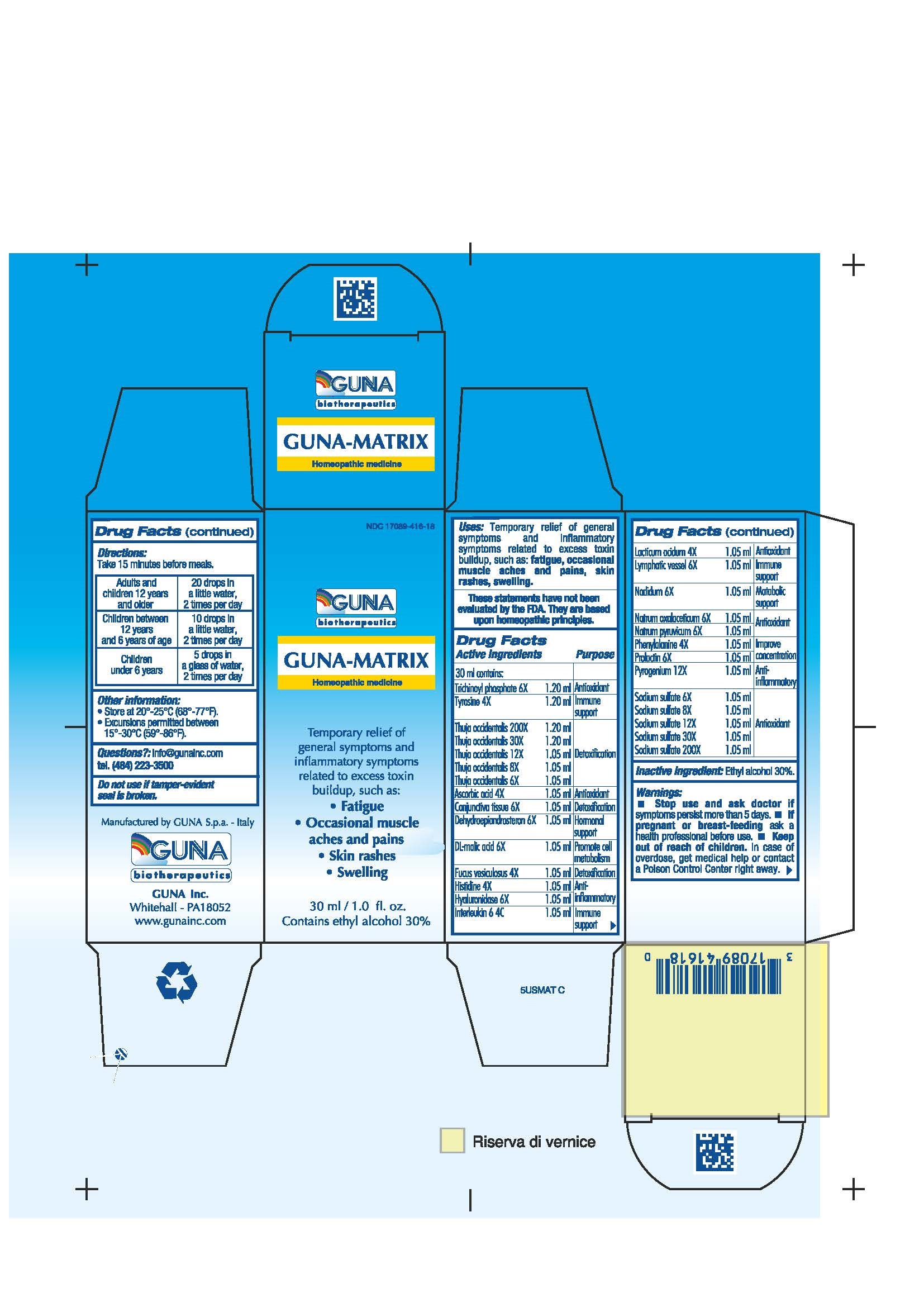
Label: GUNA-MATRIX (ascorbic acid - dodecahydroxycyclohexane dihydrate - fucus vesiculosus - histidine monohydrochloride - human interleukin-6- nonglycosylated - hyaluronidase - lactic acid, dl - malic acid - nadide - phenylalanine - prasterone - prolactin - rancid beef - sodium diethyl oxalacetate - sodium pyruvate - sodium sulfate - sus scrofa conjunctiva - sus scrofa small intestine mucosa lymph follicle - thuja occidentalis twig - tyrosine - solution/ drops
- NDC Code(s): 17089-416-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSE
ASCORBIC ACID 4X ANTIOXIDANT
CONJUNCTIVA TISSUE 6X DETOXIFICATION
DEHYDROEPIANDROSTERON 6X HORMONAL SUPPORT
DL-MALIC ACID 6X PROMOTE CELL METABOLISM
FUCUS VESICULOSUS 4X DETOXIFICATION
HISTIDINE 4X ANTI-INFLAMMATORY
HYALURONIDASE 6X ANTI-INFLAMMATORY
INTERLEUKIN 6 4C IMMUNE SUPPORT
LACTICUM ACIDUM 4X ANTIOXIDANT
LYMPHATIC VESSEL 6X IMMUNE SUPPORT
NADIDUM 6X METABOLIC SUPPORT
NATRUM OXALACETICUM 6X ANTIOXIDANT
NATRUM PYRUVICUM 6X ANTIOXIDANT
NATRUM SULPHURICUM 6X, 8X, 12X, 30X, 200X ANTIOXIDANT
PHENYLALANINE 4X IMPROVE CONCENTRATION
PROLACTIN 6X IMPROVE CONCENTRATION
PYROGENIUM 12X ANTI-INFLAMMATORY
THUJA OCCIDENTALIS 6X, 8X, 12X, 30X, 200X DETOXIFICATION
TRICHINOYL PHOSPHATE 6X ANTIOXIDANT
TYROSINE 4X IMMUNE SUPPORT - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-MATRIX
ascorbic acid - dodecahydroxycyclohexane dihydrate - fucus vesiculosus - histidine monohydrochloride - human interleukin-6 (nonglycosylated) - hyaluronidase - lactic acid, dl - malic acid - nadide - phenylalanine - prasterone - prolactin - rancid beef - sodium diethyl oxalacetate - sodium pyruvate - sodium sulfate - sus scrofa conjunctiva - sus scrofa small intestine mucosa lymph follicle - thuja occidentalis twig - tyrosine - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-416 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 4 [hp_X] in 30 mL SUS SCROFA CONJUNCTIVA (UNII: W61ME6Q717) (SUS SCROFA CONJUNCTIVA - UNII:W61ME6Q717) SUS SCROFA CONJUNCTIVA 6 [hp_X] in 30 mL PRASTERONE (UNII: 459AG36T1B) (PRASTERONE - UNII:459AG36T1B) PRASTERONE 6 [hp_X] in 30 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 6 [hp_X] in 30 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 4 [hp_X] in 30 mL HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) (HISTIDINE - UNII:4QD397987E) HISTIDINE MONOHYDROCHLORIDE 4 [hp_X] in 30 mL HYALURONIDASE (UNII: 8KOG53Z5EM) (HYALURONIDASE - UNII:8KOG53Z5EM) HYALURONIDASE 6 [hp_X] in 30 mL HUMAN INTERLEUKIN-6 (NONGLYCOSYLATED) (UNII: 92QVL9080Y) (HUMAN INTERLEUKIN-6 (NONGLYCOSYLATED) - UNII:92QVL9080Y) HUMAN INTERLEUKIN-6 (NONGLYCOSYLATED) 4 [hp_C] in 30 mL LACTIC ACID, DL- (UNII: 3B8D35Y7S4) (LACTIC ACID, DL- - UNII:3B8D35Y7S4) LACTIC ACID, DL- 4 [hp_X] in 30 mL SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE (UNII: 308LM01C72) (SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - UNII:308LM01C72) SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE 6 [hp_X] in 30 mL NADIDE (UNII: 0U46U6E8UK) (NADIDE - UNII:0U46U6E8UK) NADIDE 6 [hp_X] in 30 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 6 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 6 [hp_X] in 30 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM SULFATE 12 [hp_X] in 30 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 4 [hp_X] in 30 mL PROLACTIN (UNII: 2S58538ZG2) (PROLACTIN - UNII:2S58538ZG2) PROLACTIN 6 [hp_X] in 30 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 12 [hp_X] in 30 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 12 [hp_X] in 30 mL DODECAHYDROXYCYCLOHEXANE DIHYDRATE (UNII: 5BWD2J7B4W) (DODECAHYDROXYCYCLOHEXANE - UNII:I1Z9VS3H64) DODECAHYDROXYCYCLOHEXANE DIHYDRATE 6 [hp_X] in 30 mL TYROSINE (UNII: 42HK56048U) (TYROSINE - UNII:42HK56048U) TYROSINE 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-416-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-416)