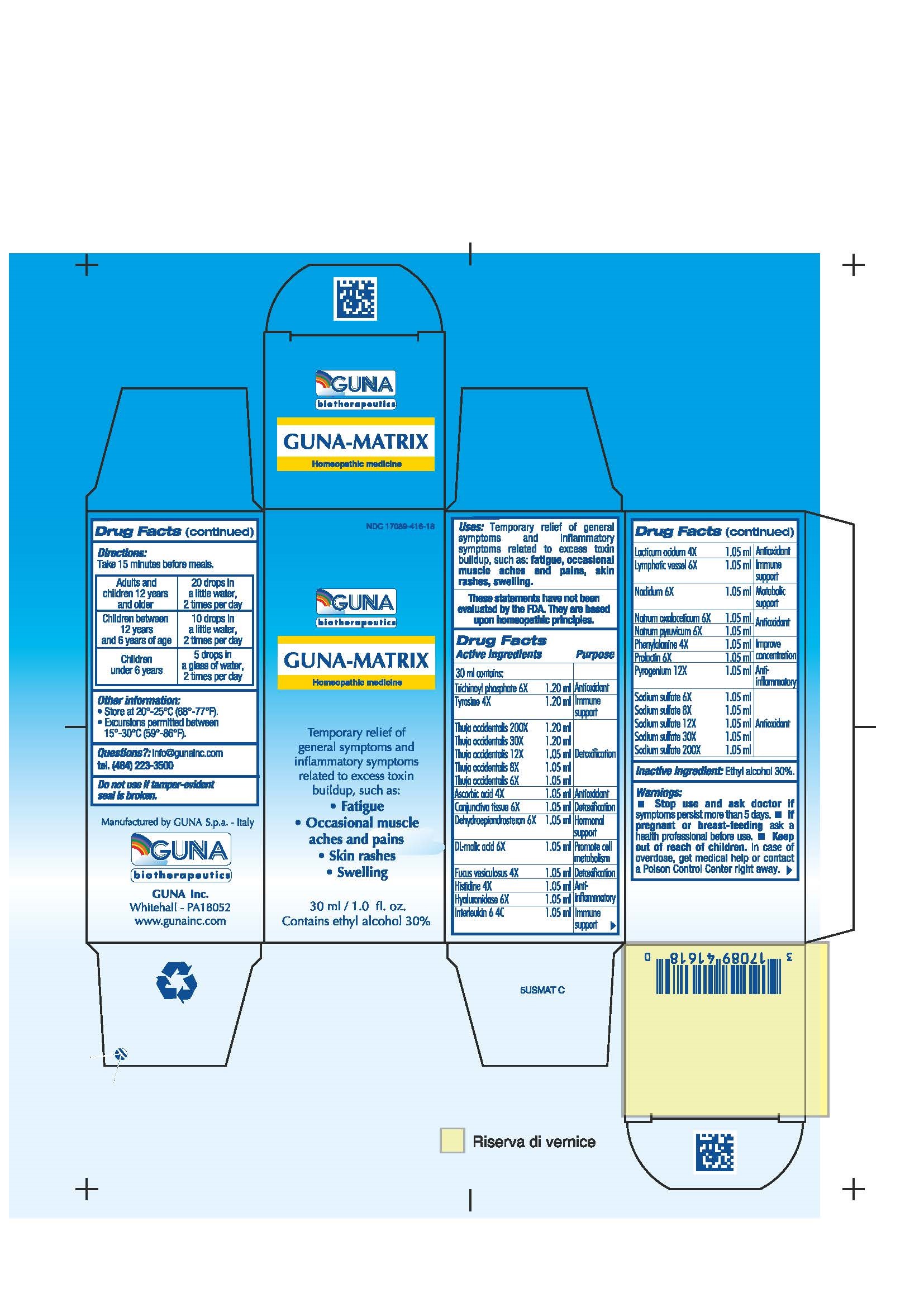
ACTIVE INGREDIENTS/PURPOSE
ASCORBIC ACID 4X ANTIOXIDANT
CONJUNCTIVA TISSUE 6X DETOXIFICATION
DEHYDROEPIANDROSTERON 6X HORMONAL SUPPORT
DL-MALIC ACID 6X PROMOTE CELL METABOLISM
FUCUS VESICULOSUS 4X DETOXIFICATION
HISTIDINE 4X ANTI-INFLAMMATORY
HYALURONIDASE 6X ANTI-INFLAMMATORY
INTERLEUKIN 6 4C IMMUNE SUPPORT
LACTICUM ACIDUM 4X ANTIOXIDANT
LYMPHATIC VESSEL 6X IMMUNE SUPPORT
NADIDUM 6X METABOLIC SUPPORT
NATRUM OXALACETICUM 6X ANTIOXIDANT
NATRUM PYRUVICUM 6X ANTIOXIDANT
NATRUM SULPHURICUM 6X, 8X, 12X, 30X, 200X ANTIOXIDANT
PHENYLALANINE 4X IMPROVE CONCENTRATION
PROLACTIN 6X IMPROVE CONCENTRATION
PYROGENIUM 12X ANTI-INFLAMMATORY
THUJA OCCIDENTALIS 6X, 8X, 12X, 30X, 200X DETOXIFICATION
TRICHINOYL PHOSPHATE 6X ANTIOXIDANT
TYROSINE 4X IMMUNE SUPPORT
USES
Temporary Relief of general symptoms and inflammatory symptoms related to excess toxin buildup, such as:
- Fatigue
- Occasionale muscle aches and pains
- Skin rashes
-
Swelling
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%