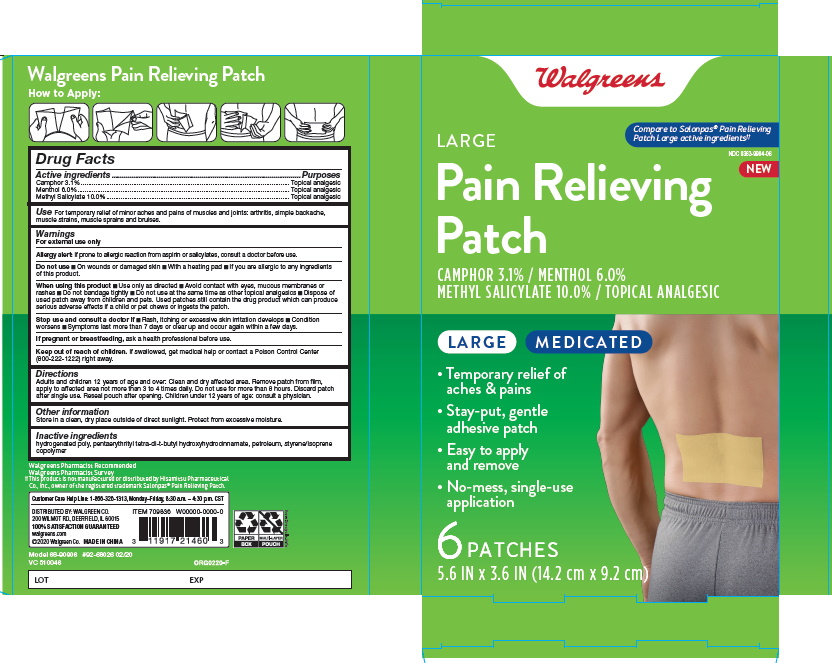
Label: LARGE PAIN RELIEVING- camphor menthol methyl salicytate patch
- NDC Code(s): 0363-9904-06
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENThydrogenated poly, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, petroleum, styrene/isoprene copolymer
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- If pregnant or breastfeeding
- PURPOSE
-
When using this product:
■ Use only as directed ■ Avoid contact with eyes, mucous membranes or rashes ■ Do not bandage tightly ■ Do not use at the same time as other topical analgesics ■ Dispose of used patch away from children and pets. Used patches still contain the drug product which can produce serious adverse effects if a child or pet chews or ingests the patch.
- Do not use
- Stop use and consult a doctor
- Storage and Care
- Pain Relieving Path
-
INGREDIENTS AND APPEARANCE
LARGE PAIN RELIEVING
camphor menthol methyl salicytate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-9904 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.1 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLY(C6-14 OLEFIN; 2 CST) (UNII: P0TX083987) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE (UNII: 44LJ2U959V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-9904-06 6 in 1 BOX 01/01/2020 1 9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2020 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(0363-9904)