Label: MITCHUM CLEAR GEL- aluminum sesquichlorohydrate liquid
-
NDC Code(s):
10967-603-22,
10967-603-34,
10967-604-22,
10967-604-34, view more10967-604-70, 10967-605-22, 10967-605-34, 10967-607-22, 10967-607-34, 10967-608-22, 10967-608-34, 10967-611-34, 10967-612-22, 10967-612-34, 10967-647-34, 10967-648-34
- Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
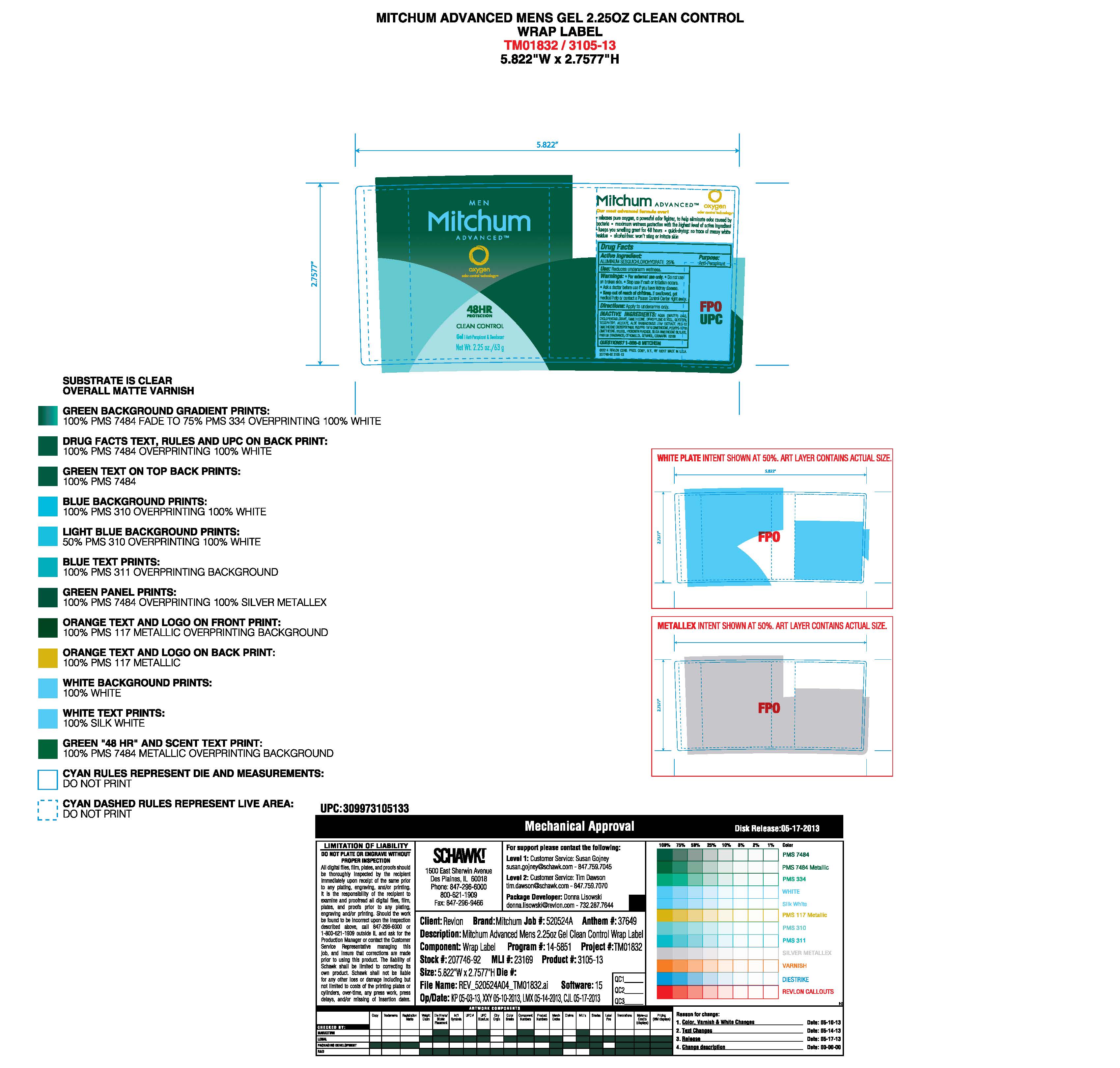
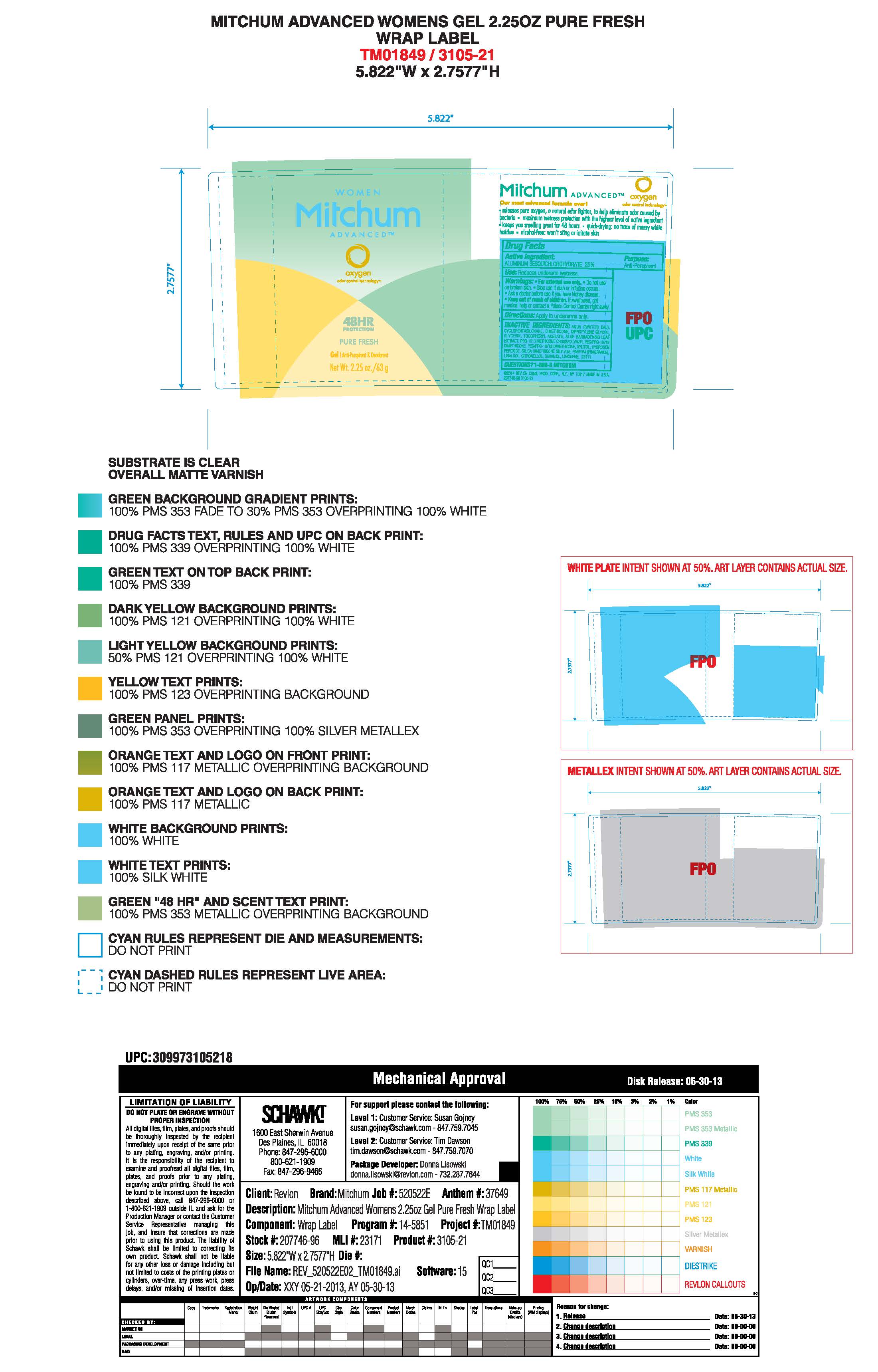
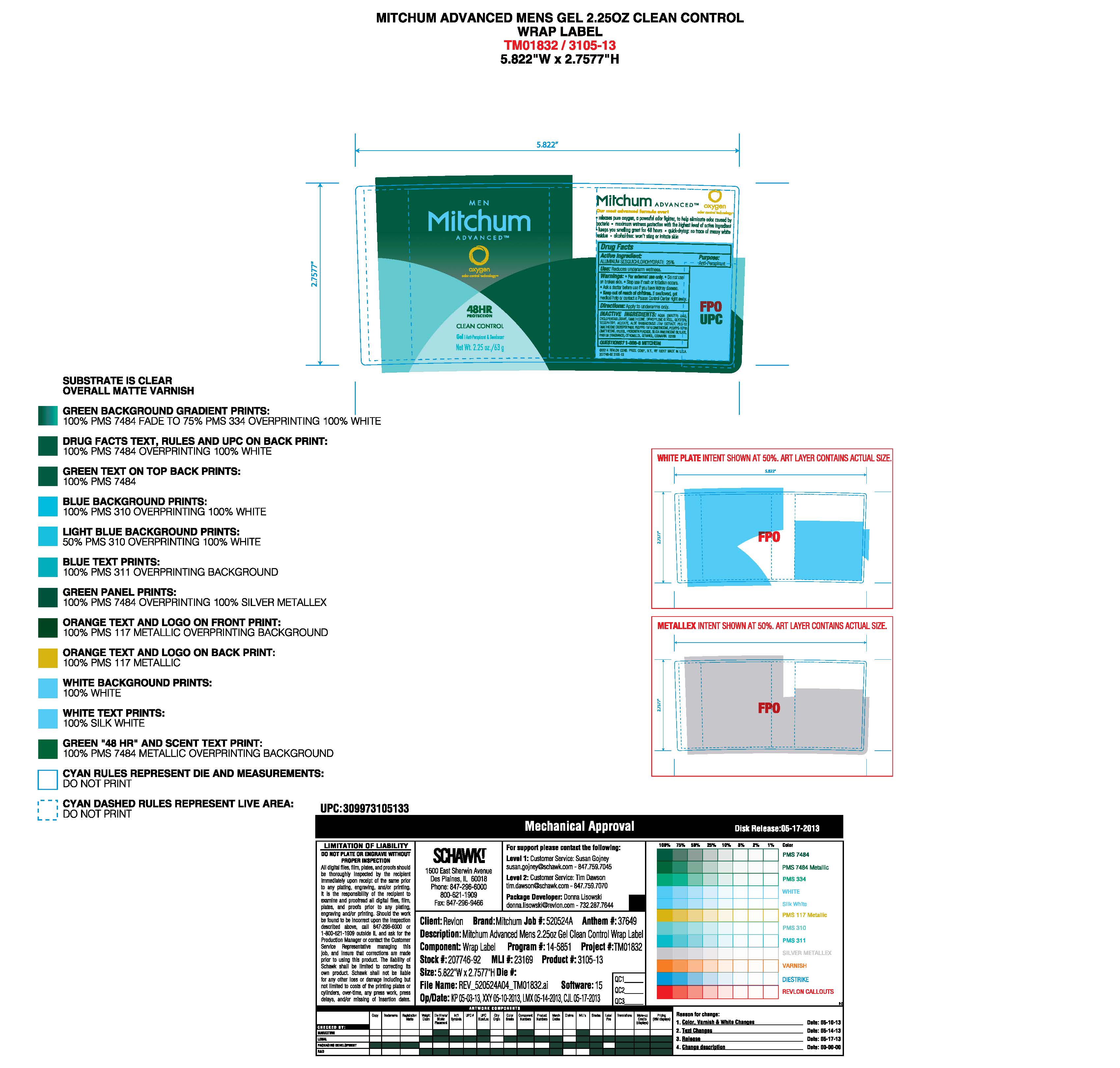
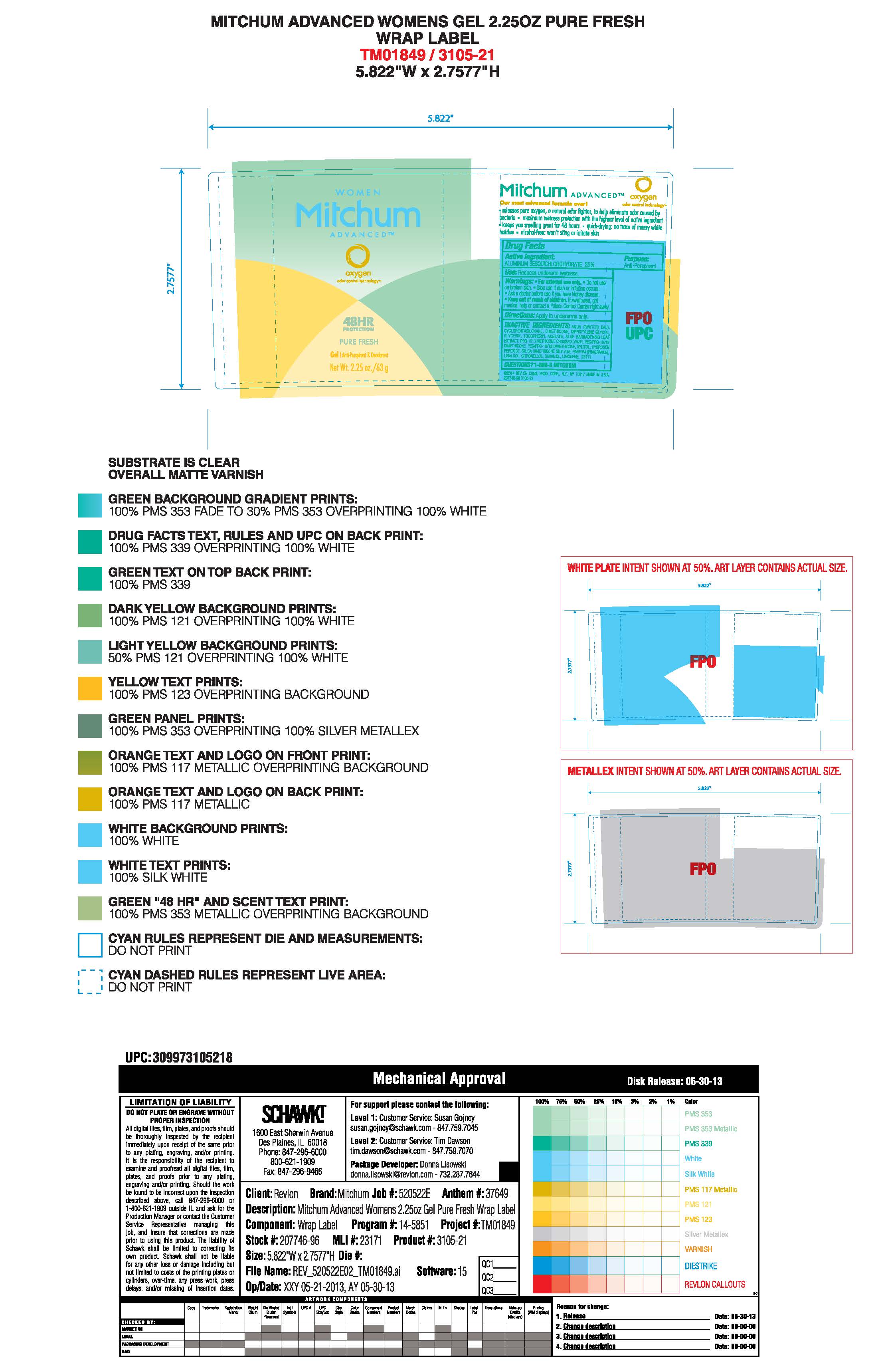
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-603-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-603-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-611-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-647 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-647-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-648 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-648-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-604 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-604-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-604-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 3 NDC:10967-604-70 20 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-605 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-605-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-605-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-607 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-607-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-607-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-608 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-608-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-608-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 MITCHUM CLEAR GEL
aluminum sesquichlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-612 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-612-34 100 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 2 NDC:10967-612-22 66 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/01/2014 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-603, 10967-604, 10967-605, 10967-607, 10967-608, 10967-611, 10967-647, 10967-612, 10967-648)