Label: FUNGUS MASTER ANTIFUNGAL TREATMENT- miconazole nitrate lotion
- NDC Code(s): 72654-001-01, 72654-001-02
- Packager: Ebanel Laboratories, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredient
-
Uses
- Proven clinically effective in the treatment of most athlete’s foot(Tinea Pedis), jock itch(Tinea Cruris) and ringworm(Tinea Corporis).
- For effective relief of itching, scaling, cracking, burning, redness, Soreness, irritation, discomfort, itchy, scaly skin between the toes, and chafing associated with jock itch.
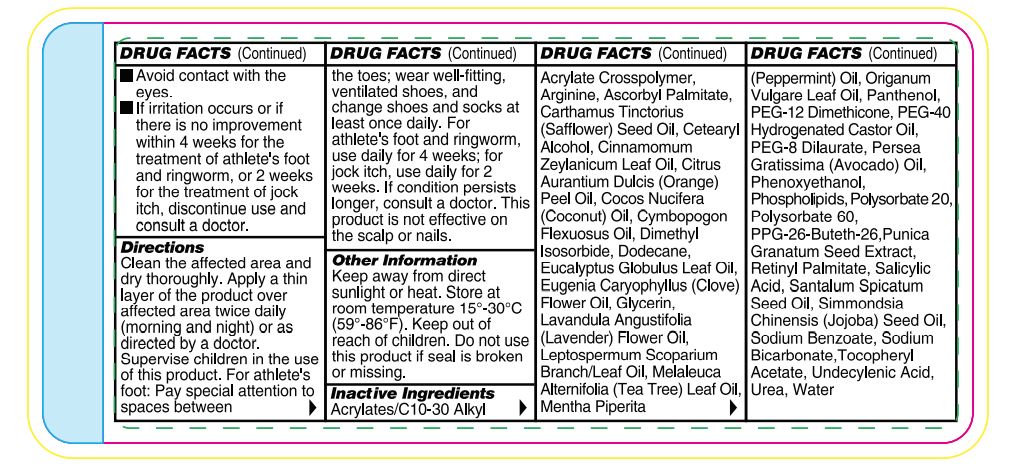
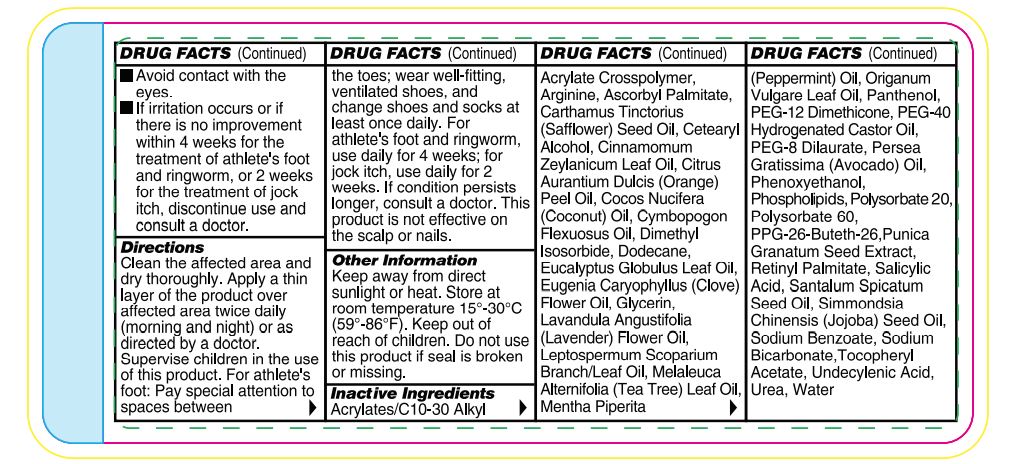
- Warnings
-
Directions
Wash the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor. Supervise children in the use of this product. For athlete’s foot: Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. For athlete’s foot and ringworm use daily for 4 weeks; for jock itch, use daily for 2 weeks. If condition persists longer, consult a doctor. This product is not effective on the scalp or nails. Store at 15-30°C (59-86°F)
-
Other Information
Functional ingredients used in this formula have been shown to improve the appearance of thickened, discolored, and unhealthylooking feet caused by dermatophytic fungi. Urea has been shown to thin, soften, and act as penetrating agent on skin. Undecylenic acid is effective as an emulsifier, cleanser, and preservative.
-
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Arginine, Ascorbyl Palmitate, Carthamus Tinctorius (Safflower) Seed Oil, Cetearyl Alcohol, Cinnamomum Zeylanicum Leaf Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Cocos Nucifera (Coconut) Oil, Cymbopogon Flexuosus Oil, Dimethyl Isosorbide, Dodecane, Eucalyptus Globulus Leaf Oil, Eugenia Caryophyllus (Clove) Flower Oil, Glycerin, Lavandula Angustifolia (Lavender) Flower Oil, Leptospermum Scoparium Branch/Leaf Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Mentha Piperita (Peppermint) Oil, Oreganum Vulgare Leaf Oil, Panthenol, PEG-12 Dimethicone, PEG-40 Hydrogenated Castor Oil, PEG-8 Dilaurate, Persea Gratissima (Avocado) Oil, Phenoxyethanol, Phospholipids, Polysorbate 20, Polysorbate 60, PPG-26-Buteth-26, Punica Granatum Seed Extract, Retinyl Palmitate, Salicylic Acid, Santalum Spicatum Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Sodium Benzoate, Sodium Bicarbonate,Tocopheryl Acetate,Undecylenic Acid, Inact ive Ingredients Urea, Water
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
FUNGUS MASTER ANTIFUNGAL TREATMENT
miconazole nitrate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72654-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ARGININE (UNII: 94ZLA3W45F) ASCORBYL PALMITATE (UNII: QN83US2B0N) SAFFLOWER OIL (UNII: 65UEH262IS) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CINNAMON LEAF OIL (UNII: S92U8SQ71V) ORANGE OIL (UNII: AKN3KSD11B) COCONUT OIL (UNII: Q9L0O73W7L) EAST INDIAN LEMONGRASS OIL (UNII: UP0M8M3VZW) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) DODECANE (UNII: 11A386X1QH) EUCALYPTUS OIL (UNII: 2R04ONI662) CLOVE OIL (UNII: 578389D6D0) GLYCERIN (UNII: PDC6A3C0OX) MANUKA OIL (UNII: M6QU9ZUH2X) TEA TREE OIL (UNII: VIF565UC2G) PEPPERMINT OIL (UNII: AV092KU4JH) PANTHENOL (UNII: WV9CM0O67Z) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PEG-8 DILAURATE (UNII: FC11NGP7E6) AVOCADO OIL (UNII: 6VNO72PFC1) PHENOXYETHANOL (UNII: HIE492ZZ3T) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYSORBATE 60 (UNII: CAL22UVI4M) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) POMEGRANATE SEED (UNII: 7294Z34NS7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SALICYLIC ACID (UNII: O414PZ4LPZ) JOJOBA OIL (UNII: 724GKU717M) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) UNDECYLENIC ACID (UNII: K3D86KJ24N) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72654-001-01 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 11/01/2019 2 NDC:72654-001-02 59 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 11/01/2019 Labeler - Ebanel Laboratories, Inc (079352161)