Uses
- Proven clinically effective in the treatment of most athlete’s foot(Tinea Pedis), jock itch(Tinea Cruris) and ringworm(Tinea Corporis).
- For effective relief of itching, scaling, cracking, burning, redness, Soreness, irritation, discomfort, itchy, scaly skin between the toes, and chafing associated with jock itch.
Warnings
- Do not use on children under 2 years of age.
- For external use only.
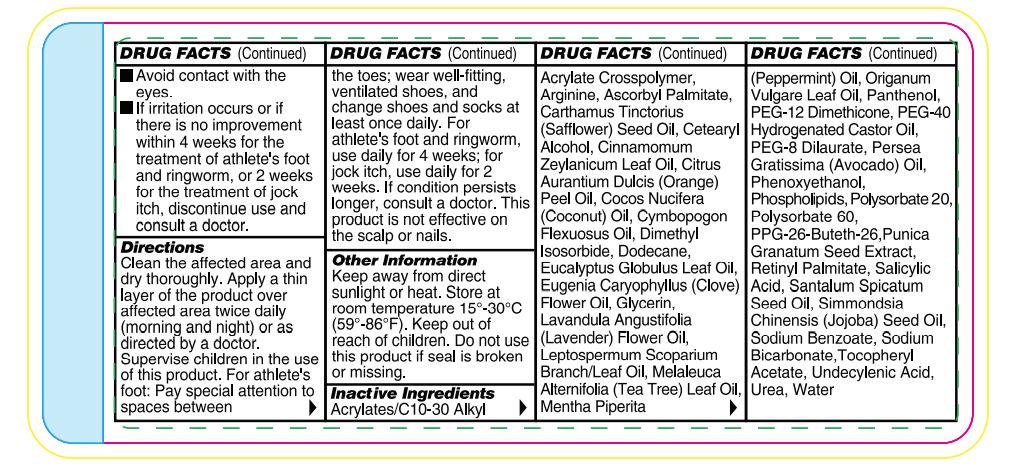
- Avoid contact with the eyes.
- If irritation occurs or if there is no improvement within 4 weeks (for athlete’s foot and ringworm) or within 2 weeks (for jock itch), discontinue use and consult a doctor.
Directions
Wash the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor. Supervise children in the use of this product. For athlete’s foot: Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. For athlete’s foot and ringworm use daily for 4 weeks; for jock itch, use daily for 2 weeks. If condition persists longer, consult a doctor. This product is not effective on the scalp or nails. Store at 15-30°C (59-86°F)
Other Information
Functional ingredients used in this formula have been shown to improve the appearance of thickened, discolored, and unhealthylooking feet caused by dermatophytic fungi. Urea has been shown to thin, soften, and act as penetrating agent on skin. Undecylenic acid is effective as an emulsifier, cleanser, and preservative.
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Arginine, Ascorbyl Palmitate, Carthamus Tinctorius (Safflower) Seed Oil, Cetearyl Alcohol, Cinnamomum Zeylanicum Leaf Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Cocos Nucifera (Coconut) Oil, Cymbopogon Flexuosus Oil, Dimethyl Isosorbide, Dodecane, Eucalyptus Globulus Leaf Oil, Eugenia Caryophyllus (Clove) Flower Oil, Glycerin, Lavandula Angustifolia (Lavender) Flower Oil, Leptospermum Scoparium Branch/Leaf Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Mentha Piperita (Peppermint) Oil, Oreganum Vulgare Leaf Oil, Panthenol, PEG-12 Dimethicone, PEG-40 Hydrogenated Castor Oil, PEG-8 Dilaurate, Persea Gratissima (Avocado) Oil, Phenoxyethanol, Phospholipids, Polysorbate 20, Polysorbate 60, PPG-26-Buteth-26, Punica Granatum Seed Extract, Retinyl Palmitate, Salicylic Acid, Santalum Spicatum Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Sodium Benzoate, Sodium Bicarbonate,Tocopheryl Acetate,Undecylenic Acid, Inact ive Ingredients Urea, Water