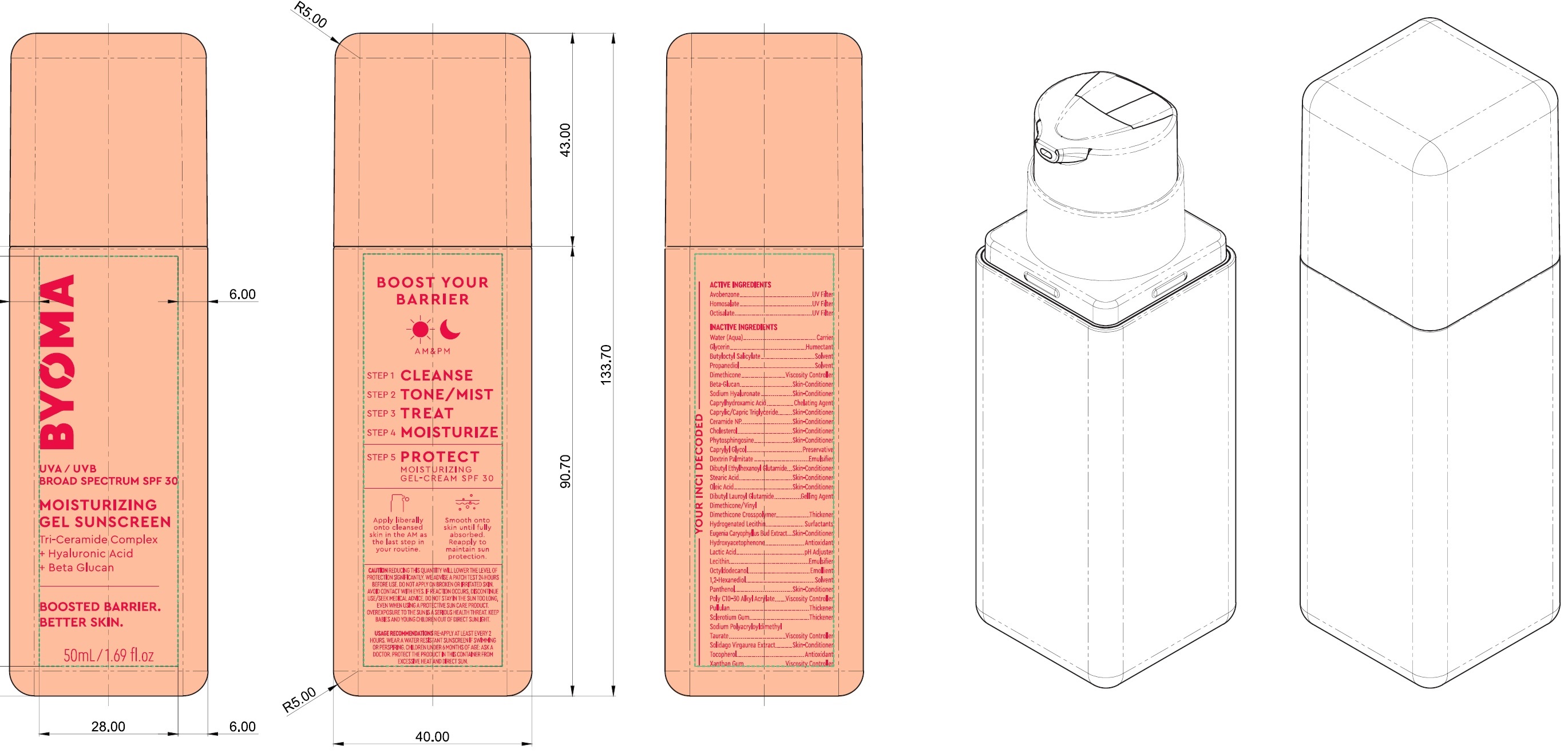
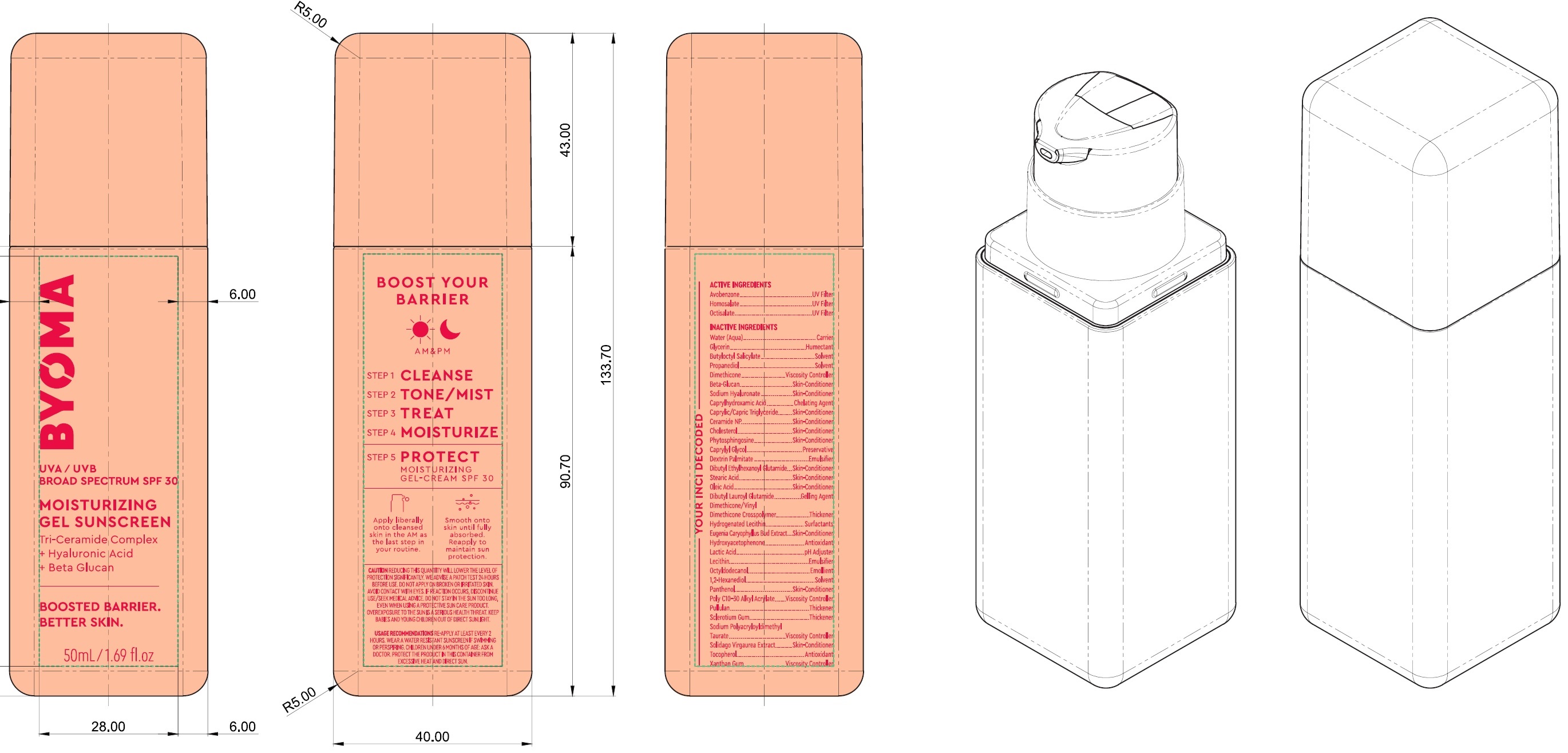
Label: BYOMA MOISTURIZING GEL SUNSCREEN BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate cream
- NDC Code(s): 14268-123-01
- Packager: ENGLEWOOD LAB, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
• Apply generously and evenly 15 minutes before sun exposure. • Use a water resistant sunscreen if swimming or sweating: • Reapply at least every 2 hours. • Children under 6 months of age: ask a doctor. • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sunprotection measures including • Limit time in the sun, especially from 10 a.m.– 2 p.m - Wear long sleeved shirts, pants, hats and sunglasses.
Sun Protection Measures - Other information
-
Inactive Ingredients
Water (Aqua), Glycerin, Butyloctyl Salicylate, Propanediol, Dimethicone, Beta-Glucan, Sodium Hyaluronate, Caprylhydroxamic Acid, Caprylic/Capric Trilgyceride, Ceramide NP, Cholesterol, Phytosphingosine, Caprylyl Glycol, Dextrin Palmitate, Dibutyl Ethylhexanoyl Glutamide, Stearic Acid, Oleic Acid, DIbutyl Lauroyl Glutamide, Dimethicone/Vinyl Dimethicone Crosspolymer, Hydrogenated Lecithin, Eugenia Caryophyllus (Clove) Bud Extract, Hydroxyacetophenone, Lactic Acid, Lecithin, Octyldodecanol, 1,2-Hexanediol, Panthenol, Poly C10-30 Alkyl Acrylate, Pullulan, Sclerotium Gum, Sodium Polyacryloyldimethyl Taurate, Solidago Virgaurea Extract, Tocopherol, Xanthan Gum.
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BYOMA MOISTURIZING GEL SUNSCREEN BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14268-123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) DIMETHICONE (UNII: 92RU3N3Y1O) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CERAMIDE NP (UNII: 4370DF050B) CHOLESTEROL (UNII: 97C5T2UQ7J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) STEARIC ACID (UNII: 4ELV7Z65AP) OLEIC ACID (UNII: 2UMI9U37CP) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) CLOVE (UNII: K48IKT5321) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) LACTIC ACID (UNII: 33X04XA5AT) OCTYLDODECANOL (UNII: 461N1O614Y) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PANTHENOL (UNII: WV9CM0O67Z) PULLULAN (UNII: 8ZQ0AYU1TT) BETASIZOFIRAN (UNII: 2X51AD1X3T) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14268-123-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/15/2023 Labeler - ENGLEWOOD LAB, INC. (172198223)