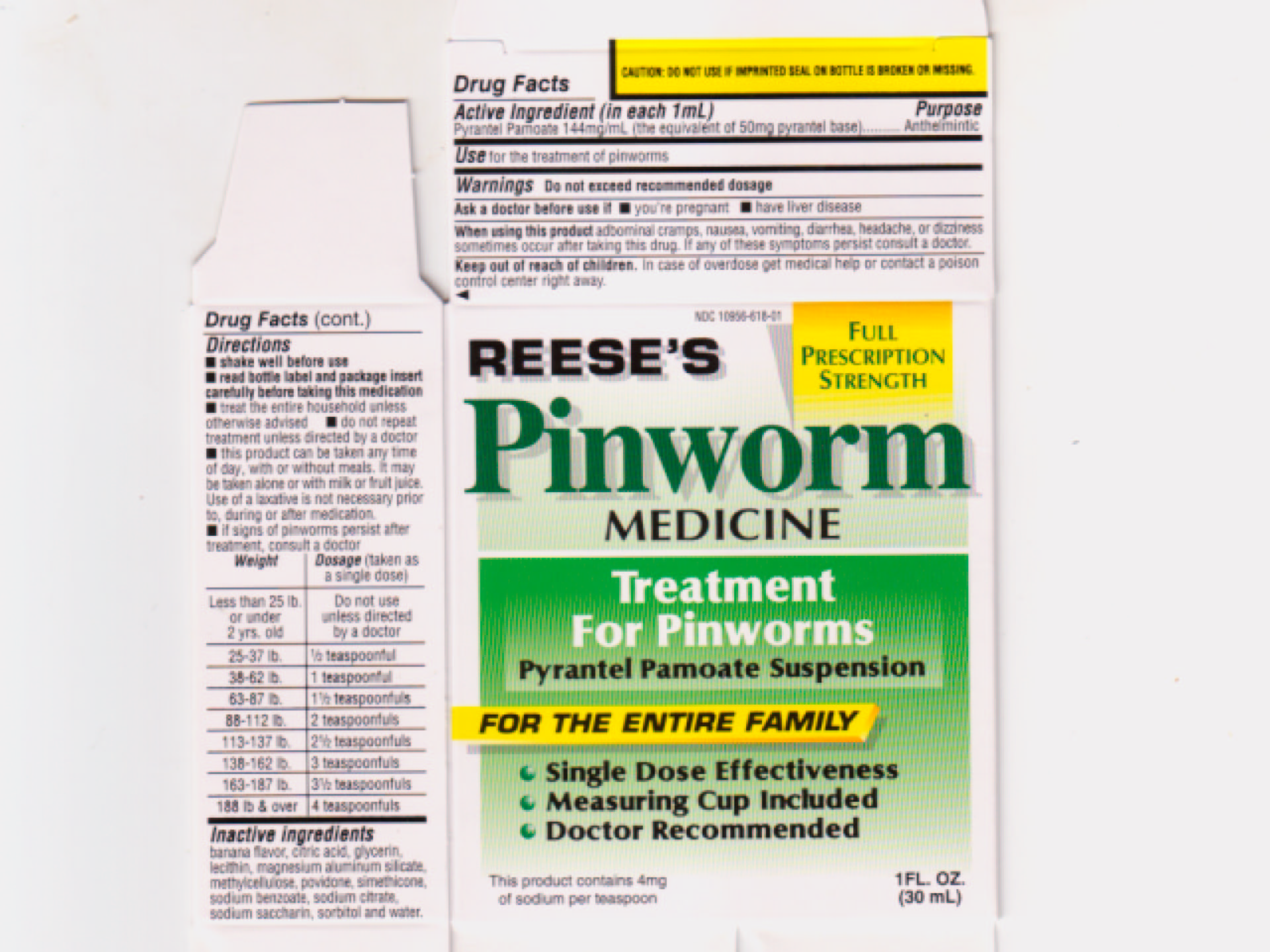
Label: PINWORM- pyrantal pamoate suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 10956-618-01 - Packager: Reese Pharmaceutical Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 3, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- shake well before use
-
read bottle label and package insert carefully before taking this medication
- treat the entire household unless otherwise advised
- do not repeat treatment unless directed by a doctor
- this product can be taken any time of day, with or without meals. It may be taken alone or with milk or fruit juice. Use of a laxative is not necessary prior to, during or after medication
- it signs of pinworms persist after treatment, consult a doctor
Weight
Dosage (taken as a single dose)
Less than 25 lb. or under 2 yrs.old
Do not use unless directed by a doctor
25-37 lb.
1/2 teaspoonful
38-62 lb.
1 teaspoonful
63-87 lb.
1 1/2 teaspoonfuls
88-112 lb.
2 teaspoonfuls
113-137 lb.
2 1/2 teaspoonfuls
138-162 lb.
3 teaspoonfuls
163-187 lb.
3 1/2 teaspoonfuls
188 lb. and over
4 teaspoonfuls
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PINWORM MEDICINE
pyrantal pamoate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10956-618 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL PAMOATE 144 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLCELLULOSE (100 CPS) (UNII: 4GFU244C4J) POVIDONE (UNII: FZ989GH94E) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor BANANA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10956-618-01 30 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part357B 01/01/2007 Labeler - Reese Pharmaceutical Company (004172052) Registrant - Elge Inc (610655136) Establishment Name Address ID/FEI Business Operations Elge Inc 610655136 manufacture