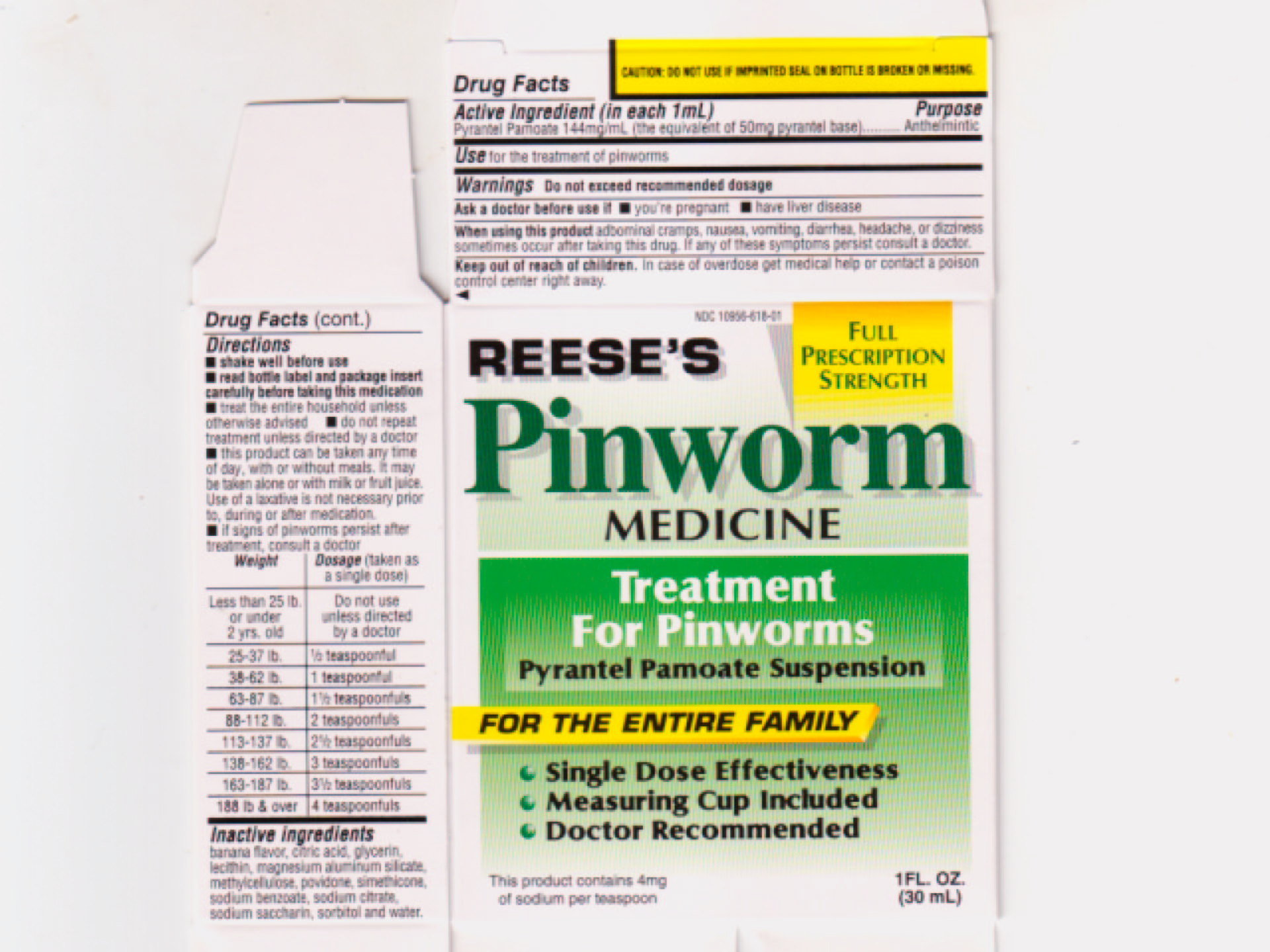
When using this product abdominal cramps, nausea, vomiting, diarrhea, headache, or dizziness sometimes occur after taking this drug. If any of these symptoms persist, consult a doctor.
Keep out of reach of children. In case of overdose get medical help or contact a poison control center right away.
Directions
- shake well before use
-
read bottle label and package insert carefully before taking this medication
- treat the entire household unless otherwise advised
- do not repeat treatment unless directed by a doctor
- this product can be taken any time of day, with or without meals. It may be taken alone or with milk or fruit juice. Use of a laxative is not necessary prior to, during or after medication
- it signs of pinworms persist after treatment, consult a doctor
| Weight
| Dosage (taken as a single dose) |
| Less than 25 lb. or under 2 yrs.old | Do not use unless directed by a doctor |
| 25-37 lb. | 1/2 teaspoonful |
| 38-62 lb. | 1 teaspoonful |
| 63-87 lb. | 1 1/2 teaspoonfuls |
| 88-112 lb. | 2 teaspoonfuls |
| 113-137 lb. | 2 1/2 teaspoonfuls |
| 138-162 lb. | 3 teaspoonfuls |
| 163-187 lb. | 3 1/2 teaspoonfuls |
| 188 lb. and over | 4 teaspoonfuls |