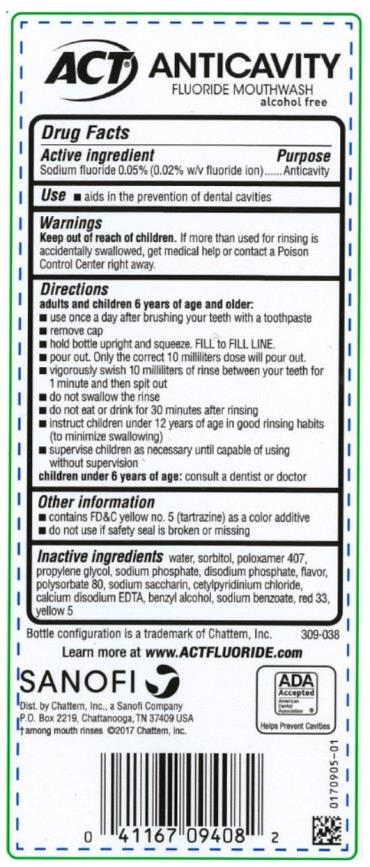
Label: ACT ANTICAVITY FLUORIDE CINNAMON- sodium fluoride rinse
- NDC Code(s): 41167-0940-8
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 6 years of age and older:
- use once a day after brushing your teeth with a toothpaste
- remove cap
- hold bottle upright and squeeze. Fill to FILL LINE
- pour out. Only the correct 10 milliliters dose will pour out.
- vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age: consult a dentist or doctor
- use once a day after brushing your teeth with a toothpaste
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACT ANTICAVITY FLUORIDE CINNAMON
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0940 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM PHOSPHATE (UNII: SE337SVY37) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM BENZOATE (UNII: OJ245FE5EU) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color red Score Shape Size Flavor CINNAMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0940-8 532 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 04/01/1994 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 832973031 manufacture(41167-0940)