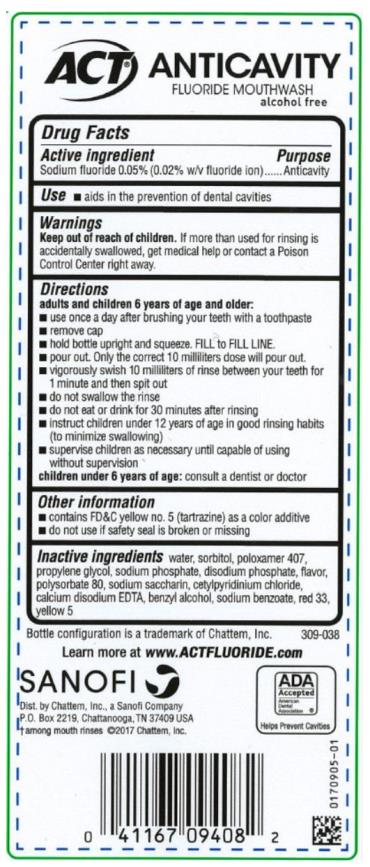
Directions
adults and children 6 years of age and older:
- use once a day after brushing your teeth with a toothpaste
- remove cap
- hold bottle upright and squeeze. Fill to FILL LINE
- pour out. Only the correct 10 milliliters dose will pour out.
- vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age: consult a dentist or doctor
Other information
- contains FD&C yellow no. 5 (tartrazine) as a color additive
- do not use if safety seal is broken or missing