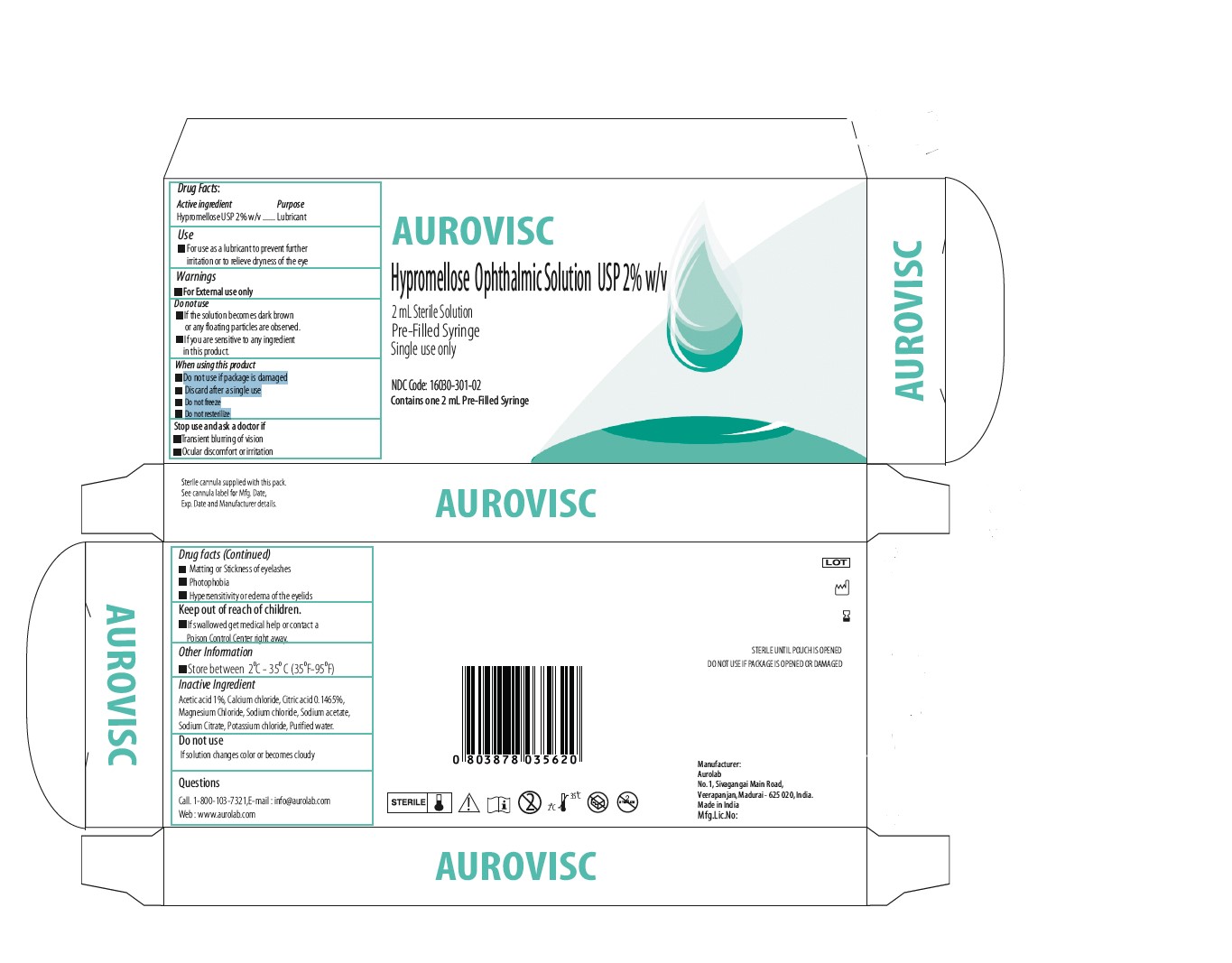
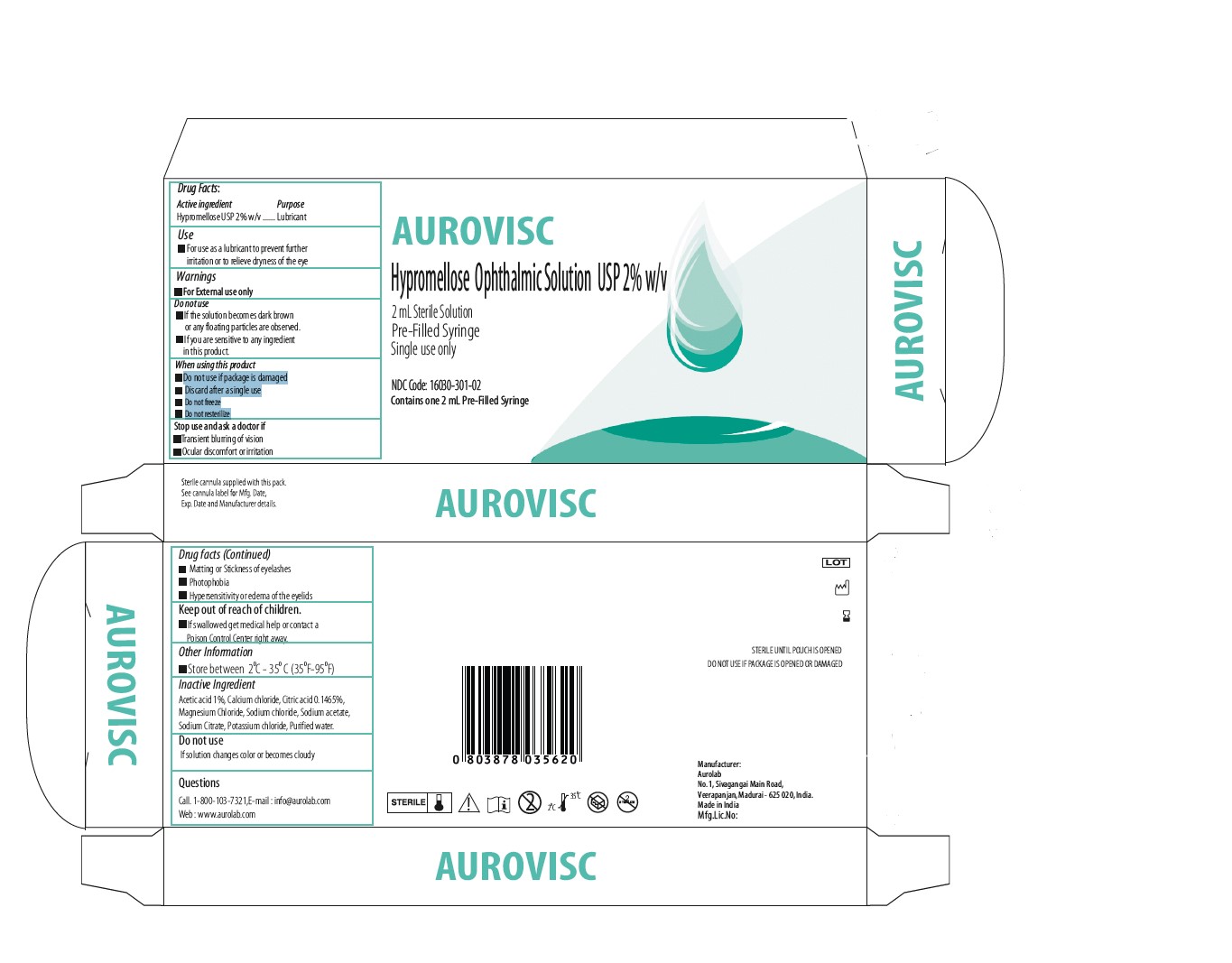
Label: AUROVISC LUBRICANT HYPROMELLOSE 2208 (15000 MPA.S) GEL/DROPS- hypromellose ophthalmic solution 2% w/v solution, gel forming / drops
- NDC Code(s): 16030-303-02
- Packager: Aurolab
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- USE
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- WARNINGS
- INDIACATIONS AND USAGE
- Purpose
- Dose
- PACKAGE CARTON
-
INGREDIENTS AND APPEARANCE
AUROVISC LUBRICANT HYPROMELLOSE 2208 (15000 MPA.S) GEL/DROPS
hypromellose ophthalmic solution 2% w/v solution, gel forming / dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16030-303 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) (HYPROMELLOSE 2208 (15000 MPA.S) - UNII:Z78RG6M2N2) HYPROMELLOSE 2208 (15000 MPA.S) 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM ACETATE (UNII: 4550K0SC9B) POTASSIUM CHLORIDE (UNII: 660YQ98I10) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) CITRIC ACID ACETATE (UNII: DSO12WL7AU) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16030-303-02 2 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package 09/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/26/2022 Labeler - Aurolab (677319965) Establishment Name Address ID/FEI Business Operations Aurolab 677319965 manufacture(16030-303)