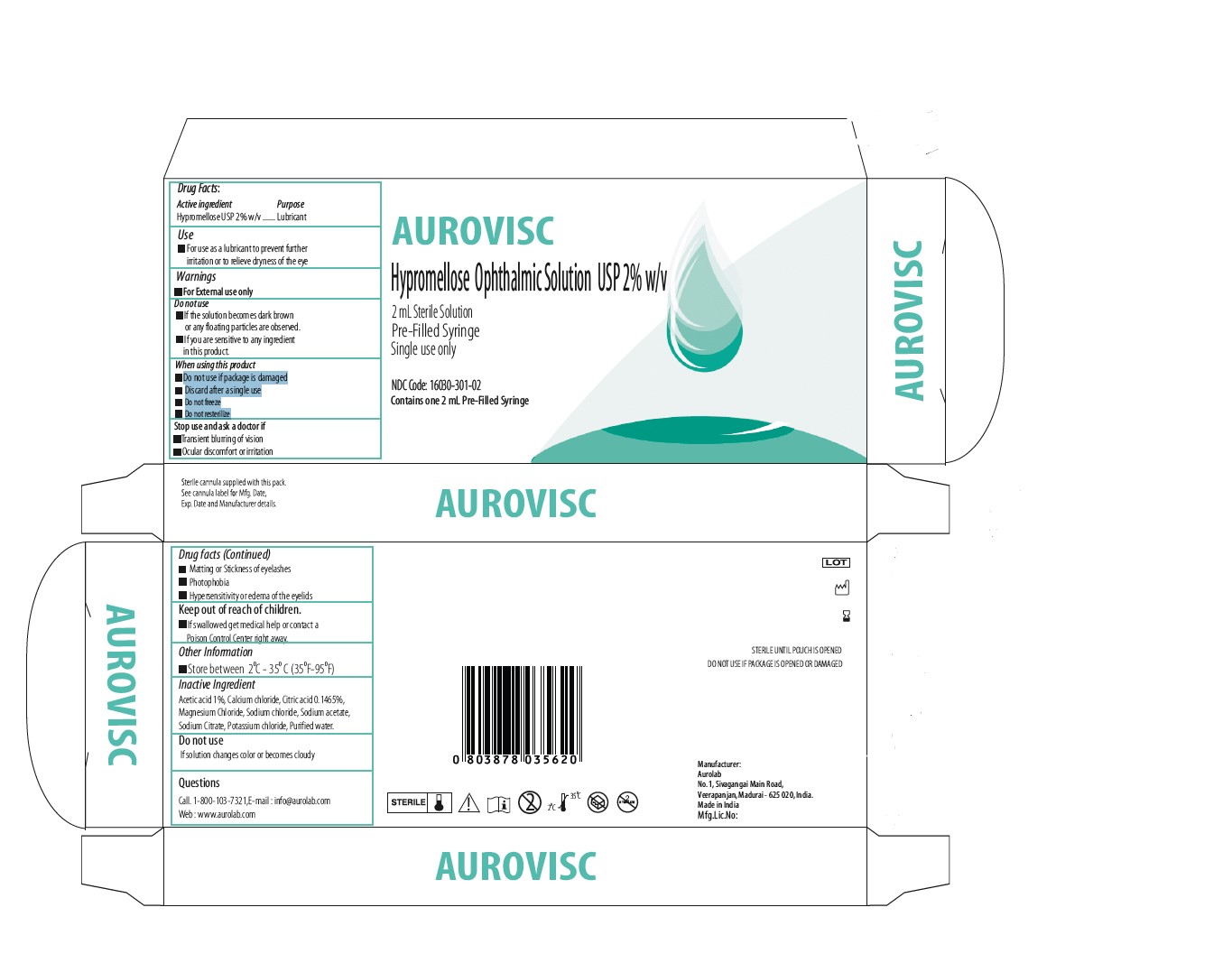
INACTIVE INGREDIENT
- Acetic acid 1%
- Calcium chloride
- Citric acid 0.1465%
- Magnesium Chloride
- Sodium chloride
- Sodium acetate,
- Sodium Citrate
- Potassium chloride
- Purified water.
KEEP OUT OF REACH OF CHILDREN
If swallowed get medical help or contact a Poison Control Center right away.
STOP USE
- Transient blurring of vision
- Ocular discomfort or irritation
- Matting or Stickness of eyelashes
- Photophobia
- Hypersensitivity or edema of the eyelids
DO NOT USE
- If the solution becomes dark brown or any floating particles are observed.
- If you are sensitive to any ingredient in this product