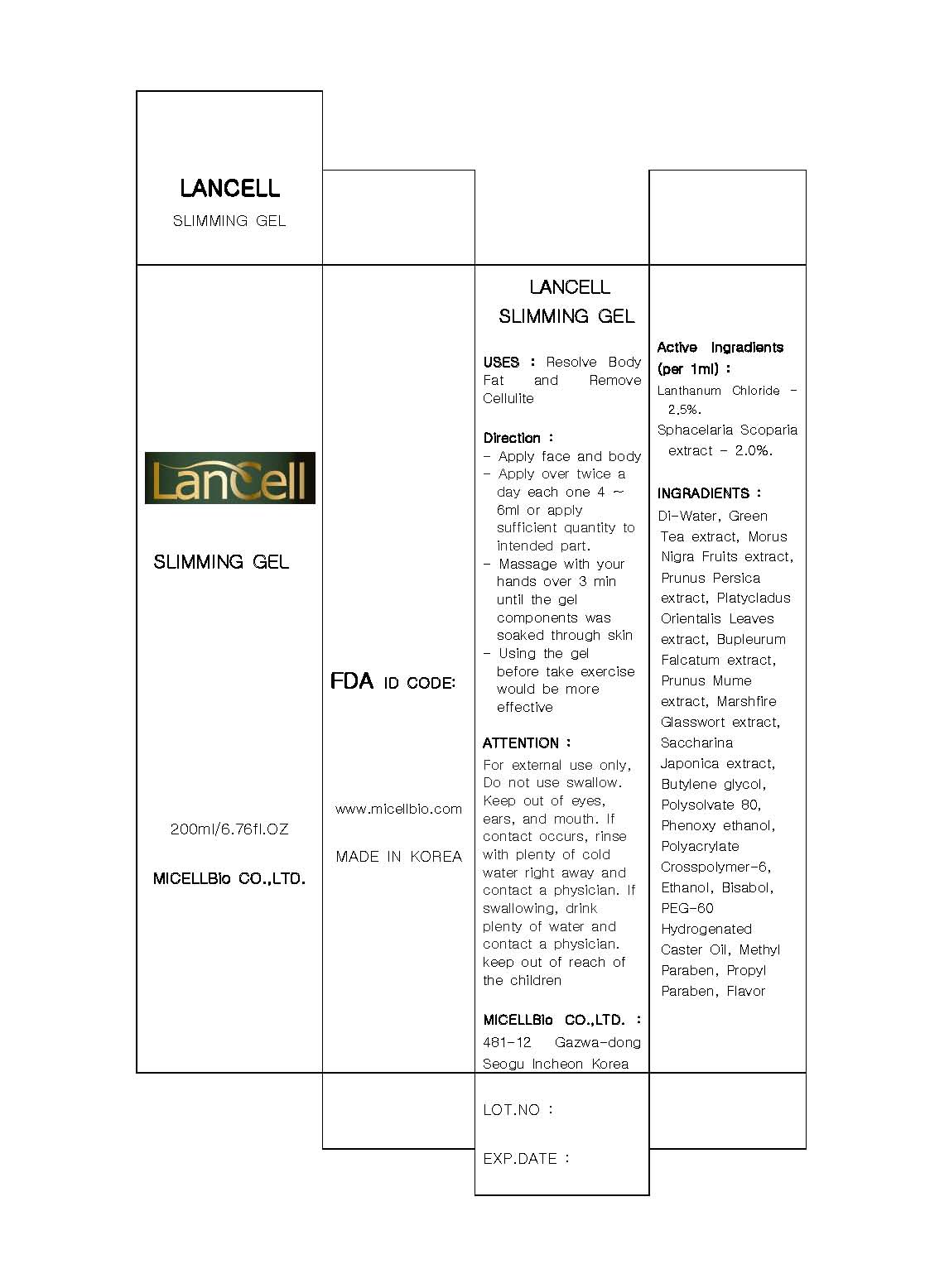
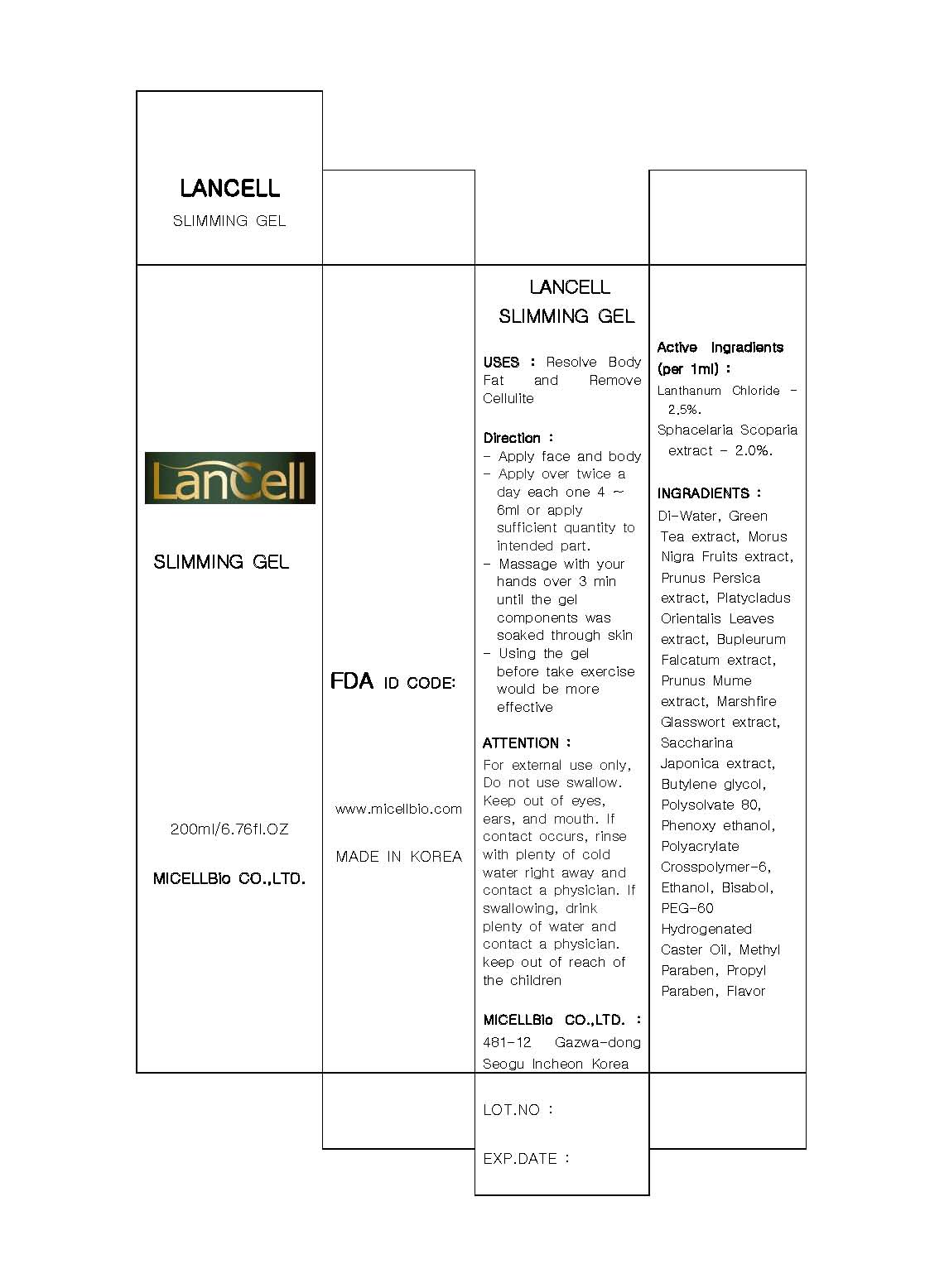
Label: LANCELL SLIMMING- lanthanum chloride gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 76084-3001-1 - Packager: MICELLBio Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 19, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
inactive ingredient: di-water, morus nigra fruits extract, pronus persica extract, platychadus orientalis extract, marchfire glasswort extract, saccharina japonica extract, butylene glycol, polysorbate 80, phenoxy ethanol, polyacrylate crosspolymer-6, ethanol, bisabol, PEG-60, hydrogenated caster oil, methyl paraben, propyl paraben, flavor
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANCELL SLIMMING
lanthanum chloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76084-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM (UNII: 6I3K30563S) (LANTHANUM - UNII:6I3K30563S) LANTHANUM 2.5 mL in 100 mL STYPOCAULON SCOPARIUM (UNII: C4AD0HDH4I) (STYPOCAULON SCOPARIUM - UNII:C4AD0HDH4I) STYPOCAULON SCOPARIUM 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYSORBATE 80 (UNII: 6OZP39ZG8H) MORUS NIGRA FRUIT (UNII: 55W745XH99) PEACH (UNII: 3OKE88I3QG) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) PRUNUS MUME FLOWER (UNII: 2N8872050J) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHANOLAMINE HYDROCHLORIDE (UNII: KKP3YYL02F) POLYETHYLENE GLYCOL 3000 (UNII: SA1B764746) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76084-3001-1 200 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/19/2010 Labeler - MICELLBio Co., Ltd (557803411) Registrant - MICELLBio Co., Ltd (557803411) Establishment Name Address ID/FEI Business Operations MICELLBio Co., Ltd 557803411 manufacture