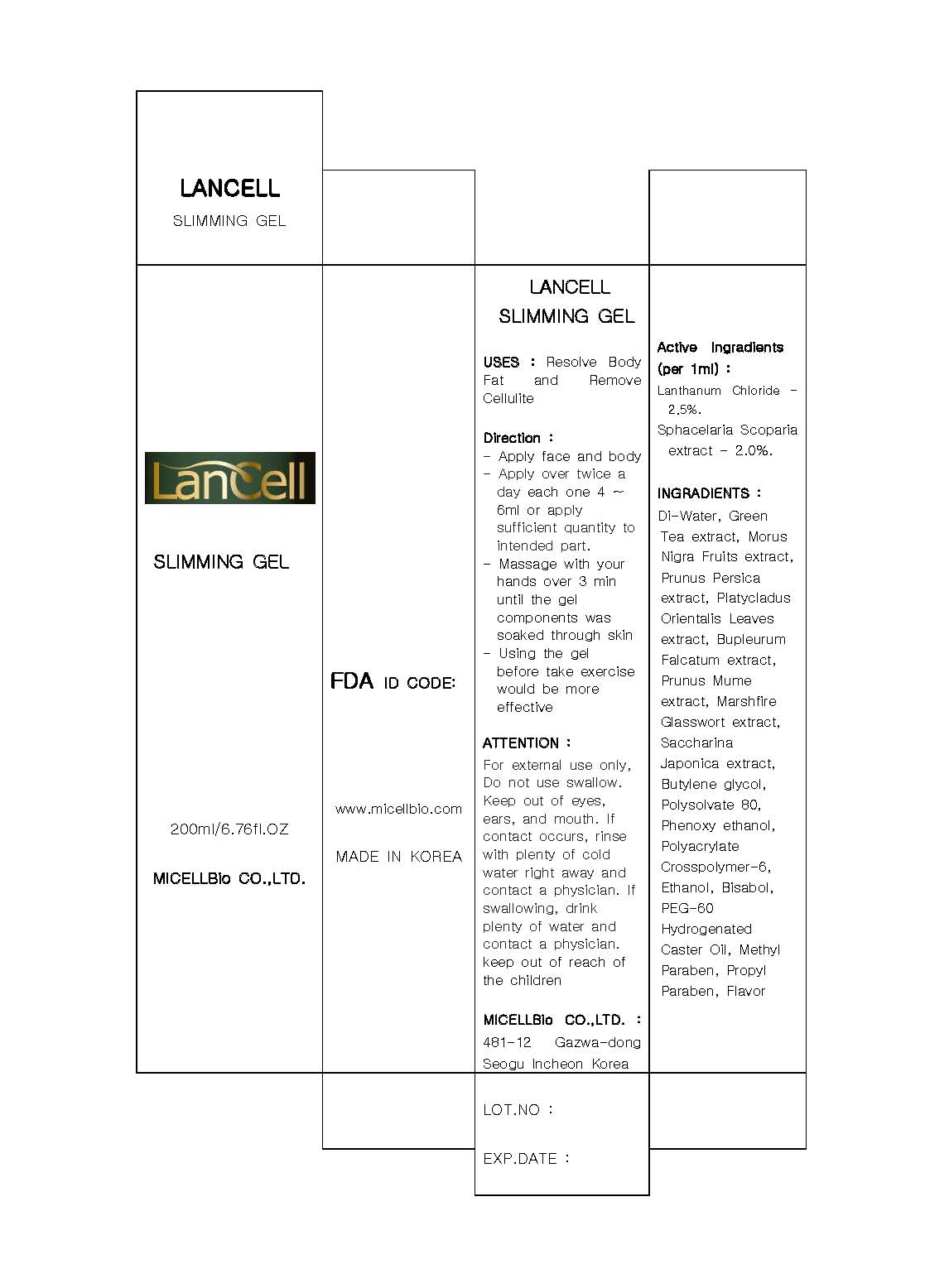
active ingredient: lanthanum chloride, sphacelaria scoparia extract
inactive ingredient: di-water, morus nigra fruits extract, pronus persica extract, platychadus orientalis extract, marchfire glasswort extract, saccharina japonica extract, butylene glycol, polysorbate 80, phenoxy ethanol, polyacrylate crosspolymer-6, ethanol, bisabol, PEG-60, hydrogenated caster oil, methyl paraben, propyl paraben, flavor
keep out of reach of the children
- apply face and body
- apply over twice a day each one 4~6 ml or apply sufficient quantity to intended part
- massage with your hands over 3 minutes until the gel components was soaked through skin
- using the gel before taking exercise would be more effective
- for external use only
- do not swallow
- keep out of eyes, ears and mouth
- remove cellulite from the body