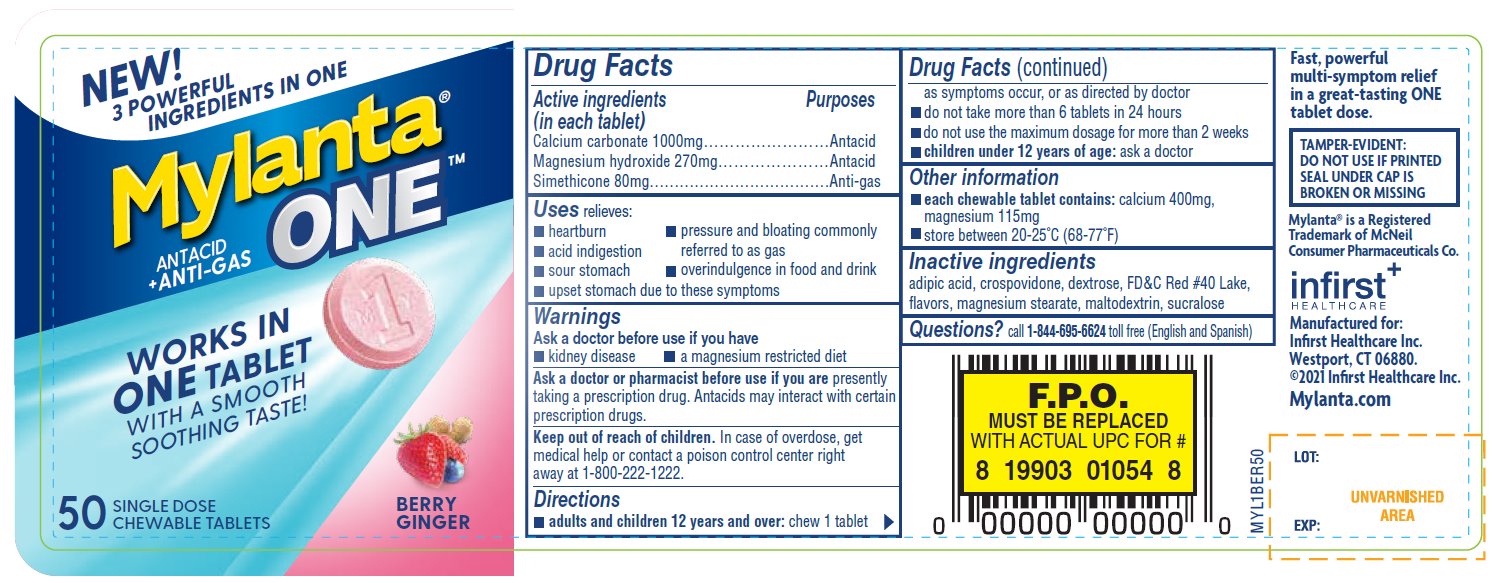
Label: MYLANTA ONE ANTACID/ANTI-GAS- calcium carbonate, magnesium hydroxide, dimethicone tablet, chewable
- NDC Code(s): 62372-552-50, 62372-552-51
- Packager: Infirst Healthcare Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each tablet)
- Purposes
- Uses
- Warnings
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
Fast, powerful multi-symptom relief in a great-tasting ONE tablet dose.
TAMPER-EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING
Mylanta ®is a Registered Trademark of McNeil Consumer Pharmaceuticals Co.
Infirst Healthcare
Manufactured for:
Infirst Healthcare Inc.
Westport, CT 06880.
©2021 Infirst Healthcare Inc.
Mylanta.com - Package/Label Principal Display Panel
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MYLANTA ONE ANTACID/ANTI-GAS
calcium carbonate, magnesium hydroxide, dimethicone tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62372-552 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1000 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 270 mg DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 80 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) CROSPOVIDONE (UNII: 2S7830E561) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score no score Shape ROUND Size 20mm Flavor BERRY, GINGER Imprint Code M1Y Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62372-552-50 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2021 02/28/2025 2 NDC:62372-552-51 50 in 1 BOTTLE; Type 0: Not a Combination Product 08/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 05/04/2021 Labeler - Infirst Healthcare Inc. (079159739)