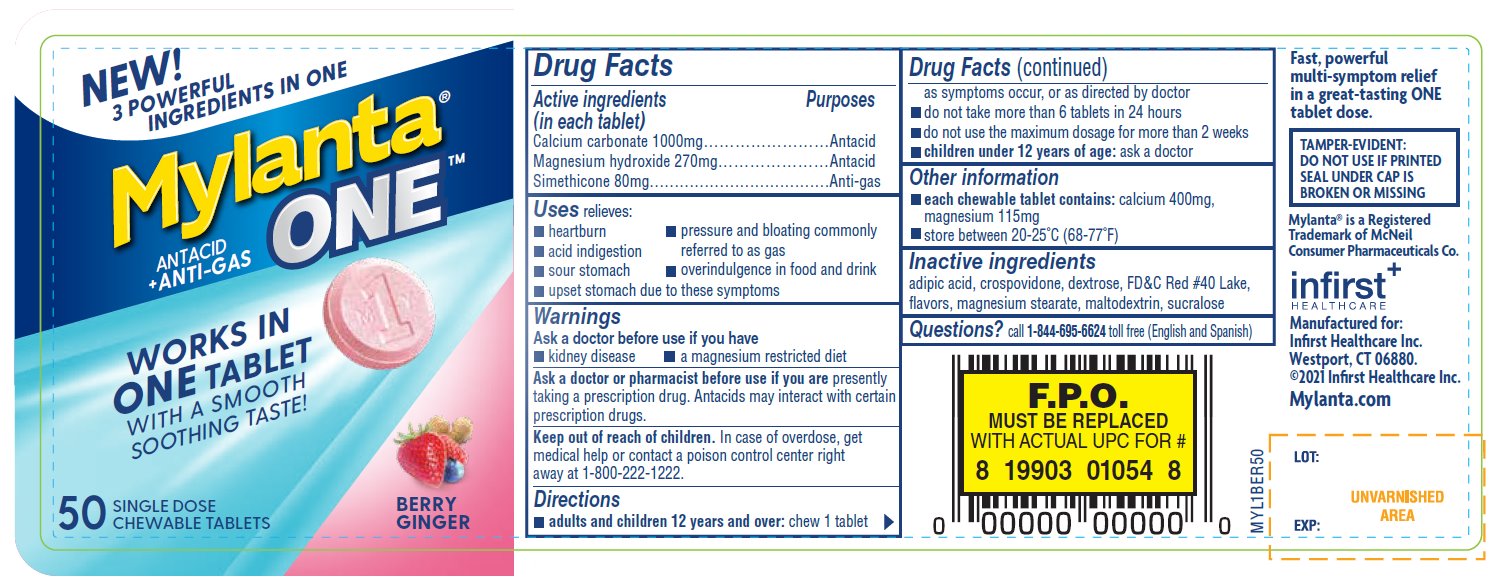
Active ingredients (in each tablet)
Calcium carbonate 1000mg
Magnesium hydroxide 270mg
Simethicone 80mg
Uses
relieves:
- heartburn
- acid indigestion
- sour stomach
- upset stomach due to these symptoms
- pressure and bloating commonly referred to as gas
- overindulgence in food and drink
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
Ask a doctor or pharmacist before use if you arepresently taking a prescription drug. Antacids may interact with certain prescription drugs.
Keep out of reach of children
In case of overdose, get medical help or contact a poison control center right away at 1-800-222-1222.
Directions
- adults and children 12 years and over:chew 1 tablet as symptoms occur, or as directed by doctor
- do not take more than 6 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
- children under 12 years of age:ask a doctor
Other information
- each chewable tablet contains:calcium 400mg, magnesium 115mg
- store between 20-25°C (68-77°F)
Inactive ingredients
adipic acid, crospovidone, dextrose, FD&C Red #40 Lake, flavors, magnesium stearate, maltodextrin, sucralose
Fast, powerful multi-symptom relief in a great-tasting ONE tablet dose.
TAMPER-EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING
Mylanta ®is a Registered Trademark of McNeil Consumer Pharmaceuticals Co.
Infirst Healthcare
Manufactured for:
Infirst Healthcare Inc.
Westport, CT 06880.
©2021 Infirst Healthcare Inc.
Mylanta.com