Label: JOHNSONS BABY CARE ESSENTIALS GIFT SET- zinc oxide kit
- NDC Code(s): 69968-0061-1, 69968-0789-9
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
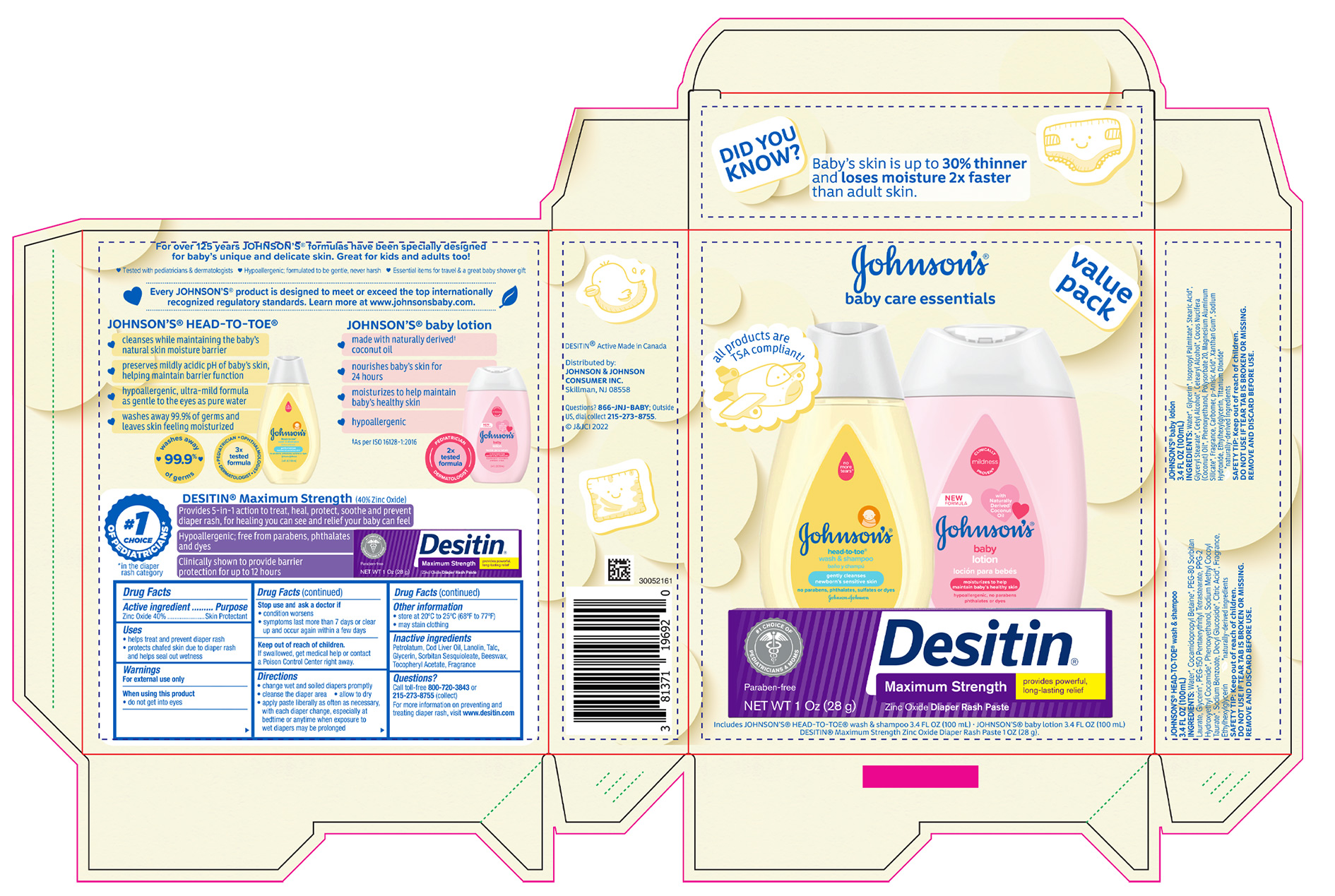
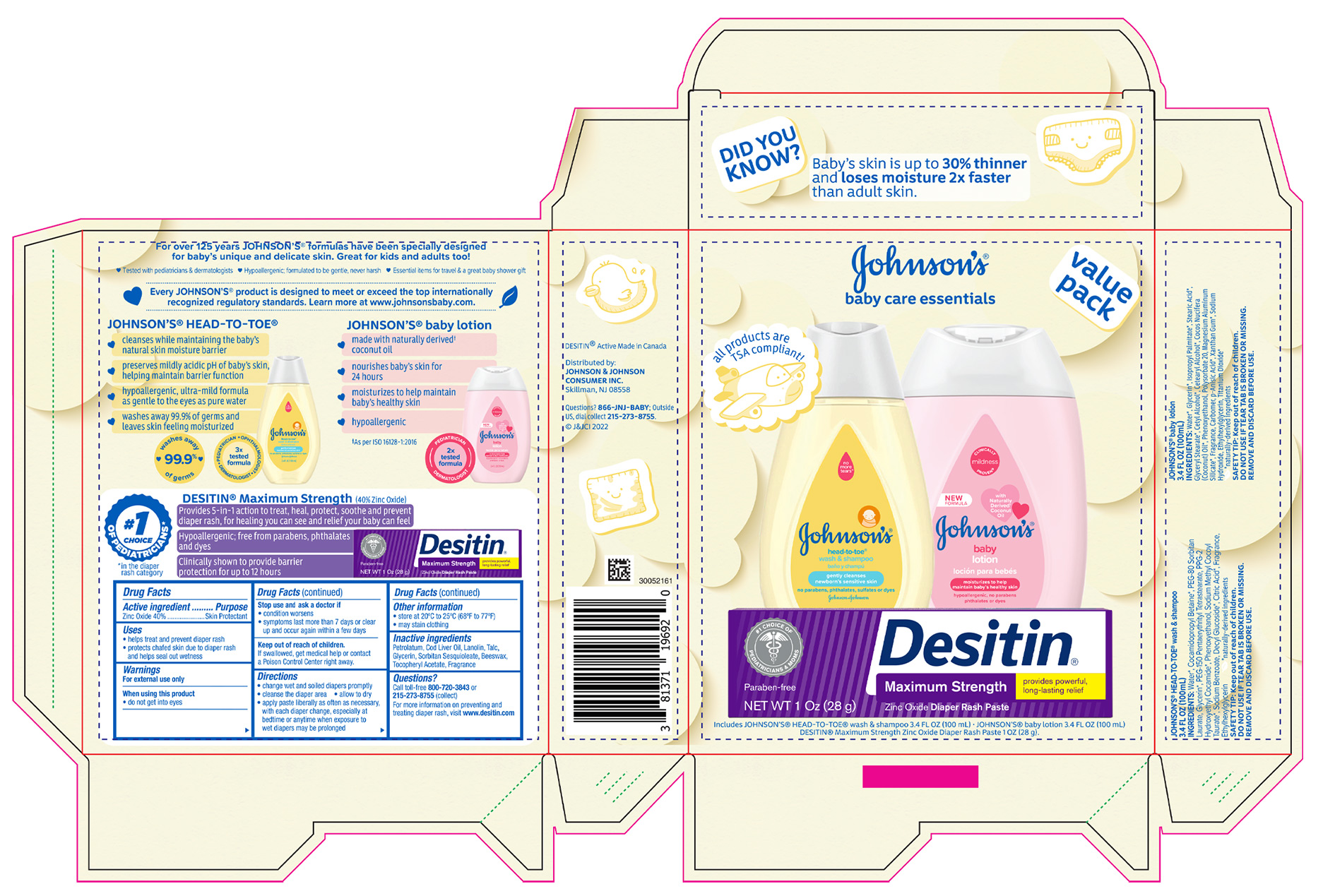
- PRINCIPAL DISPLAY PANEL - Kit Package
-
INGREDIENTS AND APPEARANCE
JOHNSONS BABY CARE ESSENTIALS GIFT SET
zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0789 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0789-9 1 in 1 PACKAGE 02/01/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 28 g Part 2 1 BOTTLE 100 mL Part 3 1 BOTTLE 100 mL Part 1 of 3 DESITIN MAXIMUM STRENGTH DIAPER RASH
zinc oxide pasteProduct Information Item Code (Source) NDC:69968-0061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 400 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) COD LIVER OIL (UNII: BBL281NWFG) LANOLIN (UNII: 7EV65EAW6H) TALC (UNII: 7SEV7J4R1U) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) YELLOW WAX (UNII: 2ZA36H0S2V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0061-1 1 in 1 CARTON 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 11/16/2015 Part 2 of 3 JOHNSONS HEAD TO TOE WASH
baby shampoos liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR PEG-80 SORBITAN LAURATE (UNII: 239B50Y732) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) INGR PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR DECYL GLUCOSIDE (UNII: Z17H97EA6Y) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 07/09/2018 Part 3 of 3 JOHNSONS BABY
lotions, oils, powders, and creams lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) INGR CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) INGR P-ANISIC ACID (UNII: 4SB6Y7DMM3) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR TITANIUM DIOXIDE (UNII: 15FIX9V2JP) INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 07/09/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 02/01/2023 Labeler - Johnson & Johnson Consumer Inc. (118772437)