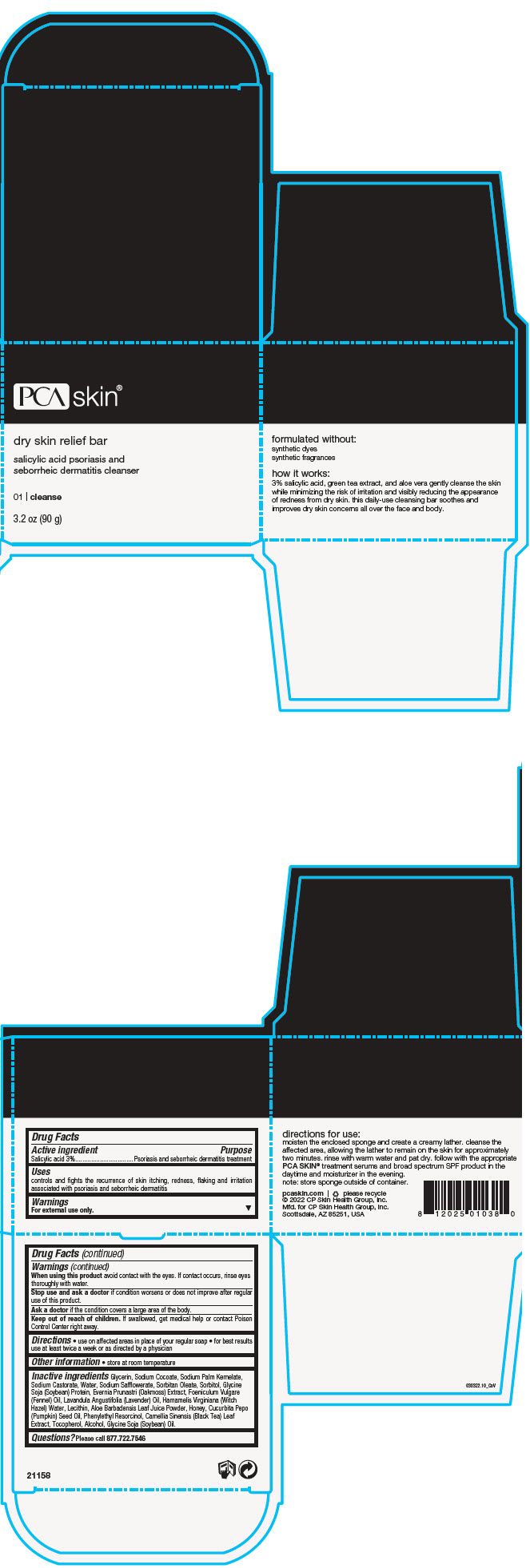
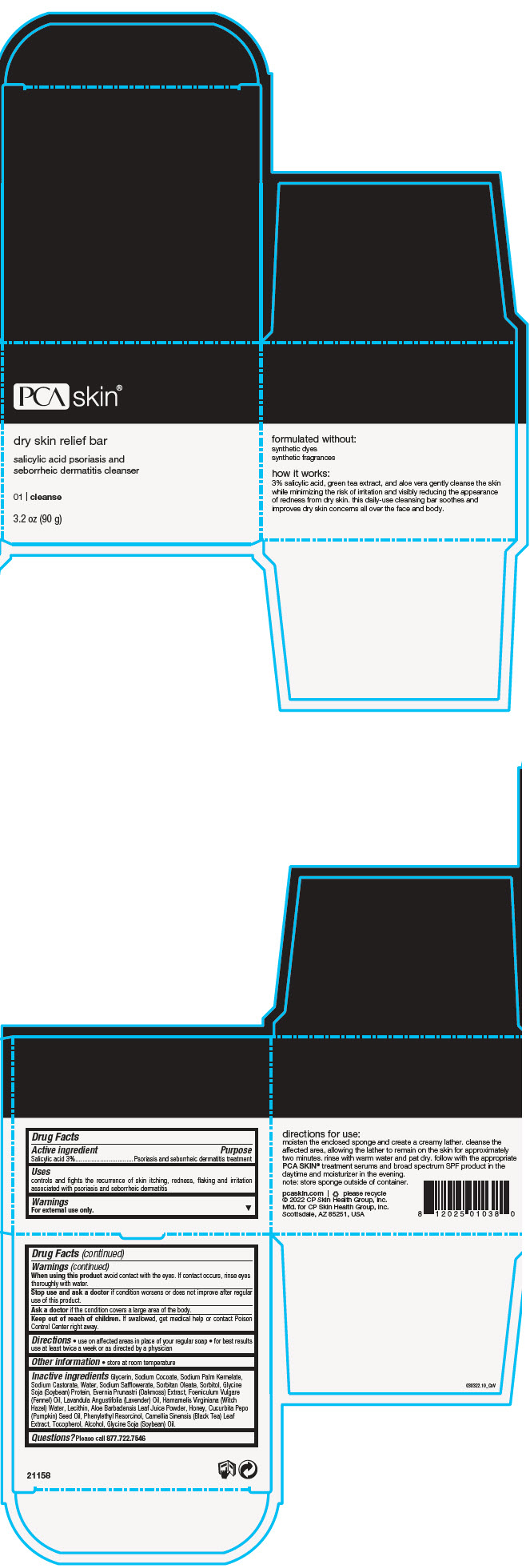
Label: DRY SKIN RELIEF BAR- salicylic acid soap
- NDC Code(s): 68726-800-01
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 19, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Glycerin, Sodium Cocoate, Sodium Palm Kernelate, Sodium Castorate, Water, Sodium Safflowerate, Sorbitan Oleate, Sorbitol, Glycine Soja (Soybean) Protein, Evernia Prunastri (Oakmoss) Extract, Foeniculum Vulgare (Fennel) Oil, Lavandula Angustifolia (Lavender) Oil, Hamamelis Virginiana (Witch Hazel) Water, Lecithin, Aloe Barbadensis Leaf Juice Powder, Honey, Cucurbita Pepo (Pumpkin) Seed Oil, Phenylethyl Resorcinol, Camellia Sinensis (Black Tea) Leaf Extract, Tocopherol, Alcohol, Glycine Soja (Soybean) Oil.
- Questions?
- PRINCIPAL DISPLAY PANEL - 90 g Jar Box
-
INGREDIENTS AND APPEARANCE
DRY SKIN RELIEF BAR
salicylic acid soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68726-800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 3 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM COCOATE (UNII: R1TQH25F4I) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) SODIUM CASTORATE (UNII: 6PAR8M5DCK) WATER (UNII: 059QF0KO0R) SODIUM SAFFLOWERATE (UNII: UHG9F87J7K) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SORBITOL (UNII: 506T60A25R) SODIUM HYDROXIDE (UNII: 55X04QC32I) FENNEL OIL (UNII: 59AAO5F6HT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LAVENDER OIL (UNII: ZBP1YXW0H8) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) ALOE VERA LEAF (UNII: ZY81Z83H0X) TEA LEAF (UNII: GH42T47V24) PUMPKIN SEED OIL (UNII: 6E5QR5USSP) HONEY (UNII: Y9H1V576FH) PHENYLETHYL RESORCINOL (UNII: G37UFG162O) SOY PROTEIN (UNII: R44IWB3RN5) ALCOHOL (UNII: 3K9958V90M) TOCOPHEROL (UNII: R0ZB2556P8) EVERNIA PRUNASTRI (UNII: O3034Q5AHK) SOYBEAN OIL (UNII: 241ATL177A) Product Characteristics Color BROWN (Dark Amber Brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68726-800-01 1 in 1 BOX 02/22/2016 1 90 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 02/22/2016 Labeler - CP Skin Health Group, Inc. (611921669)