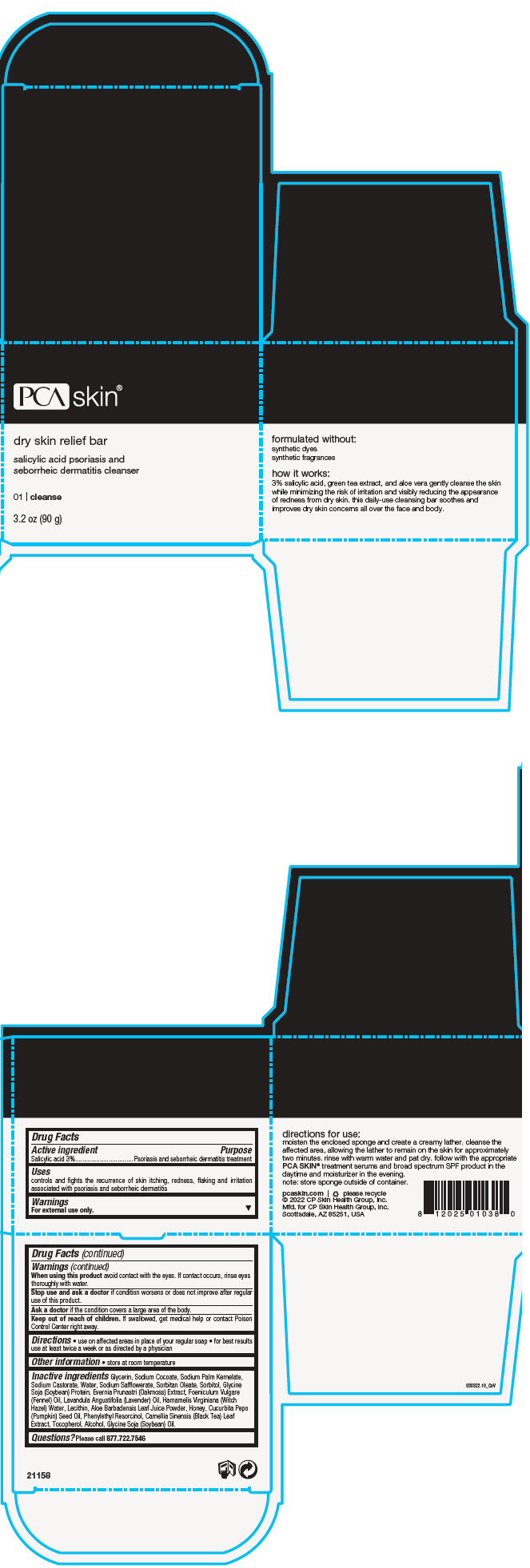
Uses
controls and fights the recurrence of skin itching, redness, flaking and irritation associated with psoriasis and seborrheic dermatitis
Warnings
For external use only.
When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- use on affected areas in place of your regular soap
- for best results use at least twice a week or as directed by a physician
Inactive ingredients
Glycerin, Sodium Cocoate, Sodium Palm Kernelate, Sodium Castorate, Water, Sodium Safflowerate, Sorbitan Oleate, Sorbitol, Glycine Soja (Soybean) Protein, Evernia Prunastri (Oakmoss) Extract, Foeniculum Vulgare (Fennel) Oil, Lavandula Angustifolia (Lavender) Oil, Hamamelis Virginiana (Witch Hazel) Water, Lecithin, Aloe Barbadensis Leaf Juice Powder, Honey, Cucurbita Pepo (Pumpkin) Seed Oil, Phenylethyl Resorcinol, Camellia Sinensis (Black Tea) Leaf Extract, Tocopherol, Alcohol, Glycine Soja (Soybean) Oil.