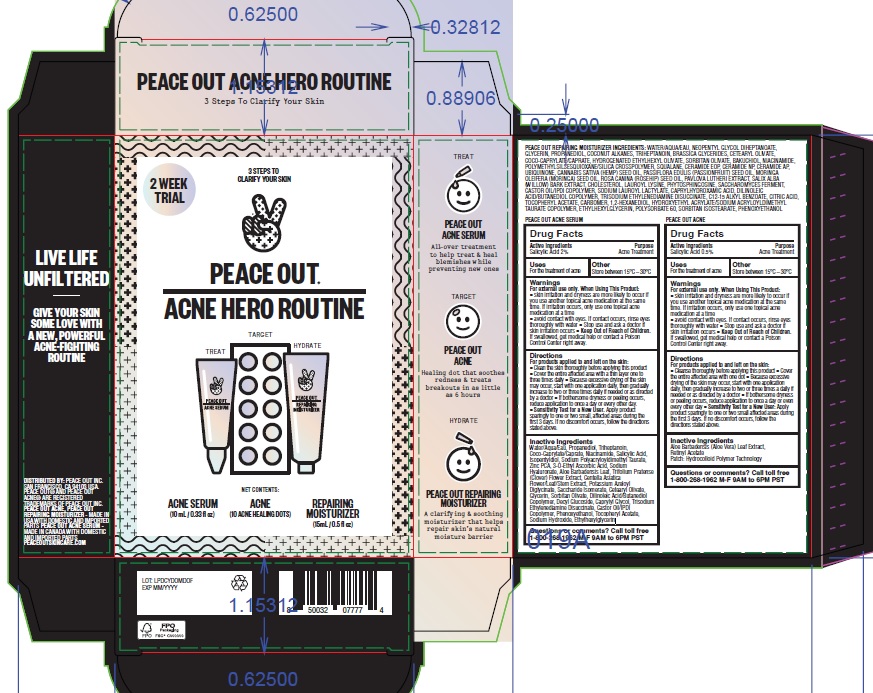
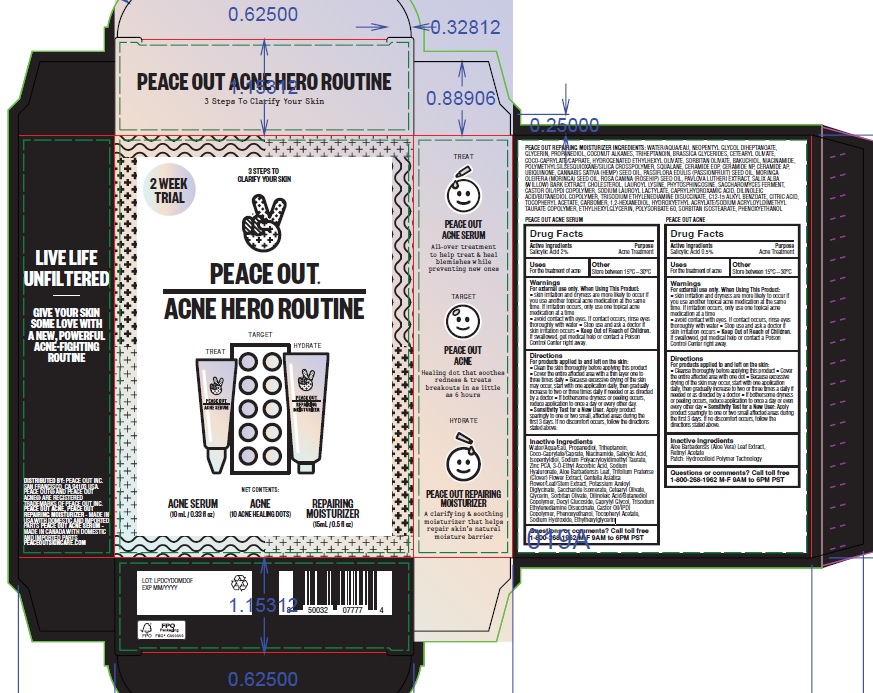
Label: PEACE OUT ACNE HERO ROUTINE- salicylic acid kit
- NDC Code(s): 71494-005-01
- Packager: Peace Out Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Other
- Warnings
-
Directions
Patch:
For products applied to and left on the skin:
- Cleanse thoroughly before applying this product
- Cover the entire affected area with one dot
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times a daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or even every other day
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
Serum:
For products applied to and left on the skin:
- Clean the skin thoroughly before applying this product
- Cover the entire affected area with a thin layer one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
-
Inactive Ingredients
Patch:
Aloe Barbadensis (Aloe Vera) Leaf Extract, Retinyl Acetate
Patch: Hydrocolloid Polymer TechnologySerum:
Water/Aqua/Eau, Propanediol, Triheptanoin, Coco-Caprylate/Caprate, Niacinamide, Salicylic Acid, Isopentyldiol, Sodium Polyacryloyldimethyl Taurate, Zinc PCA, 3-O-Ethyl Ascorbic Acid, Sodium Hyaluronate, Aloe Barbadensis Leaf, Trifolium Pratense (Clover) Flower Extract, Centella Asiatica Flower/Leaf/Stem Extract, Potassium Azeloyl Diglycinate, Saccharide Isomerate, Cetearyl Olivate, Glycerin, Sorbitan Olivate, Dilinoleic Acid/Butanediol Copolymer, Decyl Glucoside, Caprylyl Glycol, Trisodium Ethylenediamine Disuccinate, Castor Oil/IPDI Copolymer, Phenoxyethanol, Tocopheryl Acetate, Sodium Hydroxide, Ethylhexylglycerin
- Questions or comments?
- Company Information
- Packaging
-
INGREDIENTS AND APPEARANCE
PEACE OUT ACNE HERO ROUTINE
salicylic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71494-005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71494-005-01 1 in 1 CARTON; Type 0: Not a Combination Product 10/10/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 15 mL Part 2 1 CONTAINER 10 Part 3 1 BOTTLE 10 mL Part 1 of 3 PEACE OUT REPAIRING MOISTURIZER
moisturizingProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR TRIHEPTANOIN (UNII: 2P6O7CFW5K) INGR TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) INGR DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR BAKUCHIOL (UNII: OT12HJU3AR) INGR SALIX ALBA BARK (UNII: 205MXS71H7) INGR HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR CETEARYL OLIVATE (UNII: 58B69Q84JO) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR CERAMIDE AP (UNII: F1X8L2B00J) INGR UBIDECARENONE (UNII: EJ27X76M46) INGR PASSIFLORA EDULIS SEED OIL (UNII: F3VOA31UHQ) INGR COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) INGR CHOLESTEROL (UNII: 97C5T2UQ7J) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) INGR SORBITAN OLIVATE (UNII: MDL271E3GR) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) INGR ROSA CANINA SEED OIL (UNII: MHT97MG5P8) INGR LAUROYL LYSINE (UNII: 113171Q70B) INGR SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) INGR CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) INGR .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) INGR CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) INGR COCONUT ALKANES (UNII: 1E5KJY107T) INGR DIACRONEMA LUTHERI (UNII: 5N7X0S4J0B) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR CERAMIDE NP (UNII: 4370DF050B) INGR CERAMIDE 1 (UNII: 5THT33P7X7) INGR CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 2 of 3 PEACE OUT ACNE
salicylic acid patchProduct Information Item Code (Source) NDC:71494-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.359 mg Inactive Ingredients Ingredient Name Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 10/10/2022 Part 3 of 3 PEACE OUT ACNE SERUM
salicylic acid lotionProduct Information Item Code (Source) NDC:71494-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ZINC PIDOLATE (UNII: C32PQ86DH4) CETEARYL OLIVATE (UNII: 58B69Q84JO) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) GLYCERIN (UNII: PDC6A3C0OX) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) SORBITAN OLIVATE (UNII: MDL271E3GR) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) CAPRYLYL GLYCOL (UNII: 00YIU5438U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) NIACINAMIDE (UNII: 25X51I8RD4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CENTELLA ASIATICA (UNII: 7M867G6T1U) SACCHARIDE ISOMERATE (UNII: W8K377W98I) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) PROPANEDIOL (UNII: 5965N8W85T) TRIHEPTANOIN (UNII: 2P6O7CFW5K) ISOPENTYLDIOL (UNII: 19NOL5474Q) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 10/10/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 10/10/2022 Labeler - Peace Out Inc. (043143413)