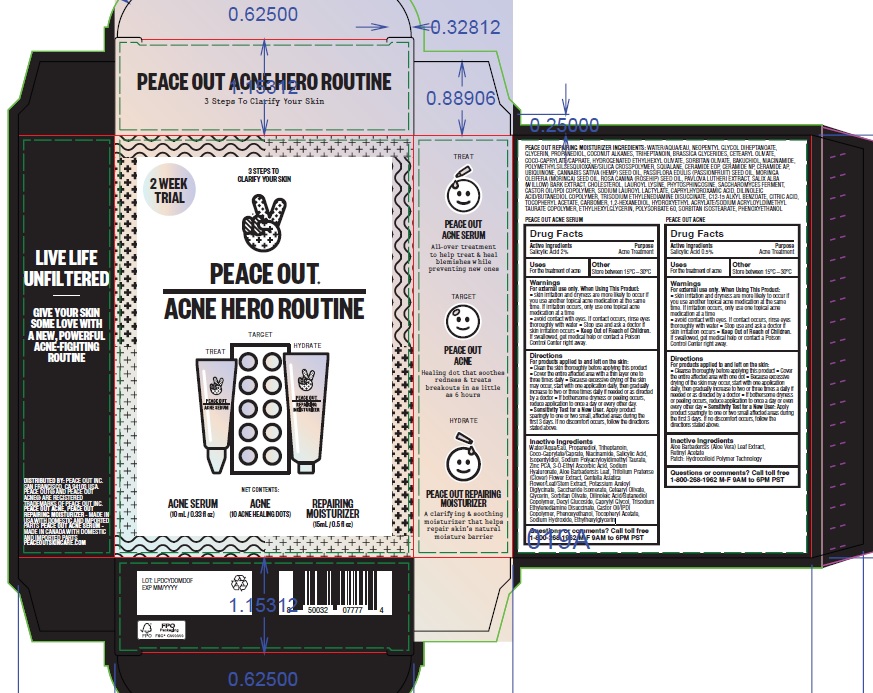
Warnings
For external use only
Directions
Patch:
For products applied to and left on the skin:
- Cleanse thoroughly before applying this product
- Cover the entire affected area with one dot
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times a daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or even every other day
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
Serum:
For products applied to and left on the skin:
- Clean the skin thoroughly before applying this product
- Cover the entire affected area with a thin layer one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
Inactive Ingredients
Patch:
Aloe Barbadensis (Aloe Vera) Leaf Extract, Retinyl Acetate
Patch: Hydrocolloid Polymer Technology
Serum:
Water/Aqua/Eau, Propanediol, Triheptanoin, Coco-Caprylate/Caprate, Niacinamide, Salicylic Acid, Isopentyldiol, Sodium Polyacryloyldimethyl Taurate, Zinc PCA, 3-O-Ethyl Ascorbic Acid, Sodium Hyaluronate, Aloe Barbadensis Leaf, Trifolium Pratense (Clover) Flower Extract, Centella Asiatica Flower/Leaf/Stem Extract, Potassium Azeloyl Diglycinate, Saccharide Isomerate, Cetearyl Olivate, Glycerin, Sorbitan Olivate, Dilinoleic Acid/Butanediol Copolymer, Decyl Glucoside, Caprylyl Glycol, Trisodium Ethylenediamine Disuccinate, Castor Oil/IPDI Copolymer, Phenoxyethanol, Tocopheryl Acetate, Sodium Hydroxide, Ethylhexylglycerin