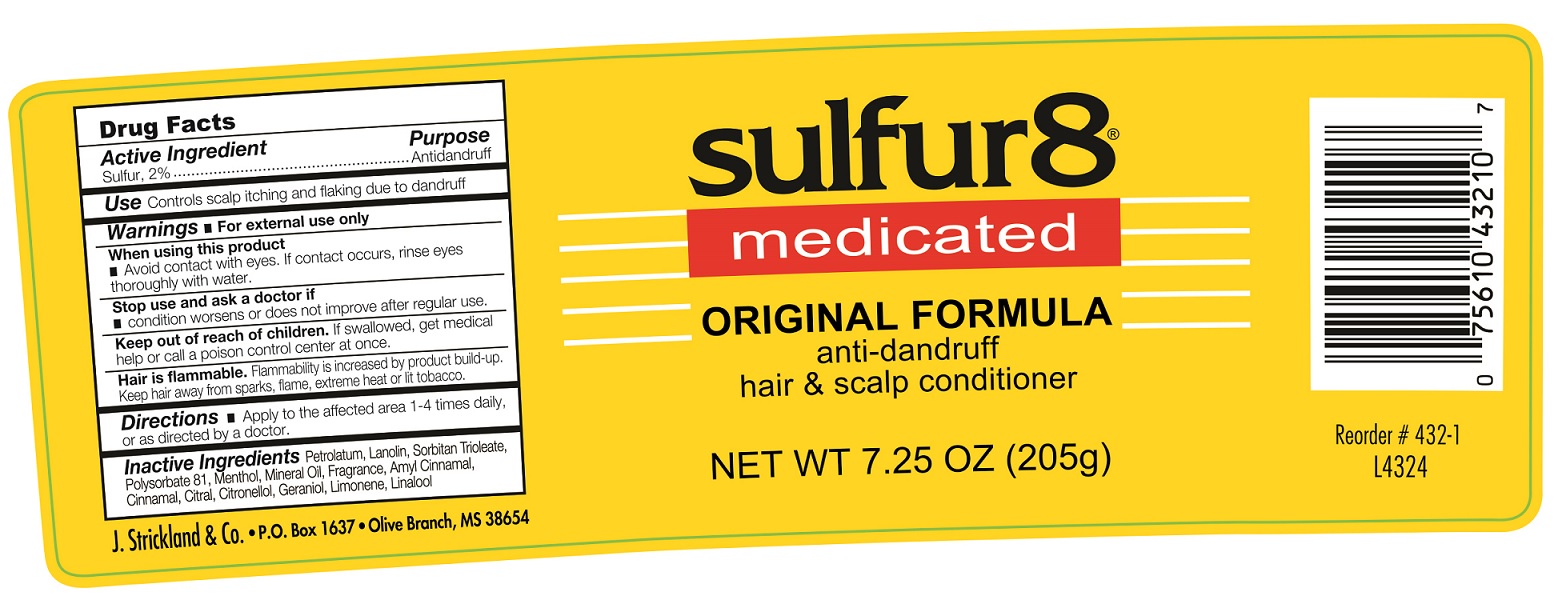
Label: SULFUR 8 ORIGINAL ANTI-DANDRUFF HAIR AND SCALP CONDITIONER- sulfur ointment
- NDC Code(s): 12022-017-00, 12022-017-01, 12022-017-02
- Packager: J. Strickland & Co.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use:
- Warnings:
- Directions
- Inactive Ingredients
- 12022-017-00
- 12022-017-01
- 12022-017-02
-
INGREDIENTS AND APPEARANCE
SULFUR 8 ORIGINAL ANTI-DANDRUFF HAIR AND SCALP CONDITIONER
sulfur ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12022-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 20 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) SORBITAN TRIOLEATE (UNII: QE6F49RPJ1) MENTHOL (UNII: L7T10EIP3A) MINERAL OIL (UNII: T5L8T28FGP) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) CINNAMALDEHYDE (UNII: SR60A3XG0F) CITRAL (UNII: T7EU0O9VPP) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12022-017-00 57 g in 1 JAR; Type 0: Not a Combination Product 12/01/1990 2 NDC:12022-017-01 113 g in 1 JAR; Type 0: Not a Combination Product 12/01/1990 3 NDC:12022-017-02 205 g in 1 JAR; Type 0: Not a Combination Product 12/01/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 12/01/1990 Labeler - J. Strickland & Co. (007023112) Registrant - J. Strickland & Co. (007023112) Establishment Name Address ID/FEI Business Operations J. Strickland & Co. 007023112 manufacture(12022-017)