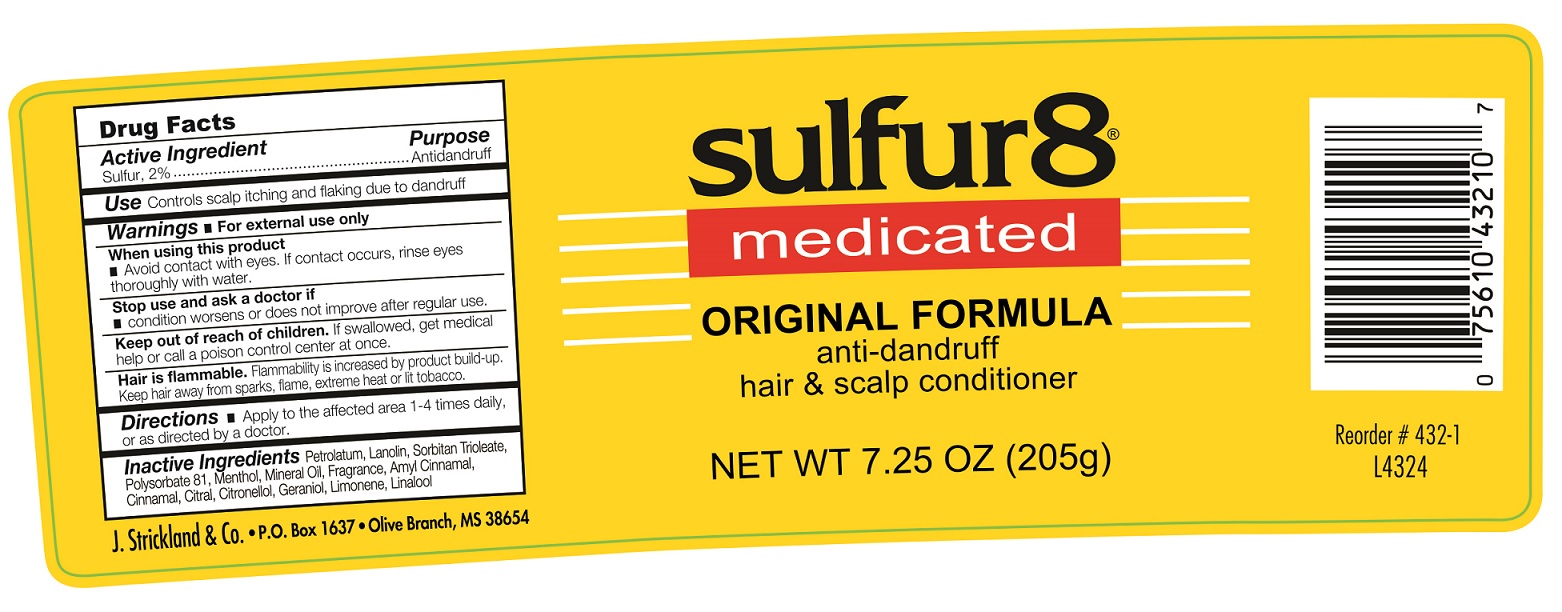
Active Ingredient
Sulfur, 2%
Use:
Controls scalp itching and flaking due to dandruff
Warnings:
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse eyes throughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve after regular use.
Keep out of reach of children
If swallower, get medical help or call a poison control center at once.
Directions
- Apply to the affected area 1-4 times daily, or as directed by a doctor.
Inactive Ingredients
Petrolatum, Landin, Sorbitan Trioleate, Pclysorbate 81, Menthol, Mineral Oil, Fragrance, Amyl Cinnamal, Cinnamal, Citral, Citronellol, Geraniol, Limonene, Linalool
12022-017-00

12022-017-01

12022-017-02
J. Strickland & Co.