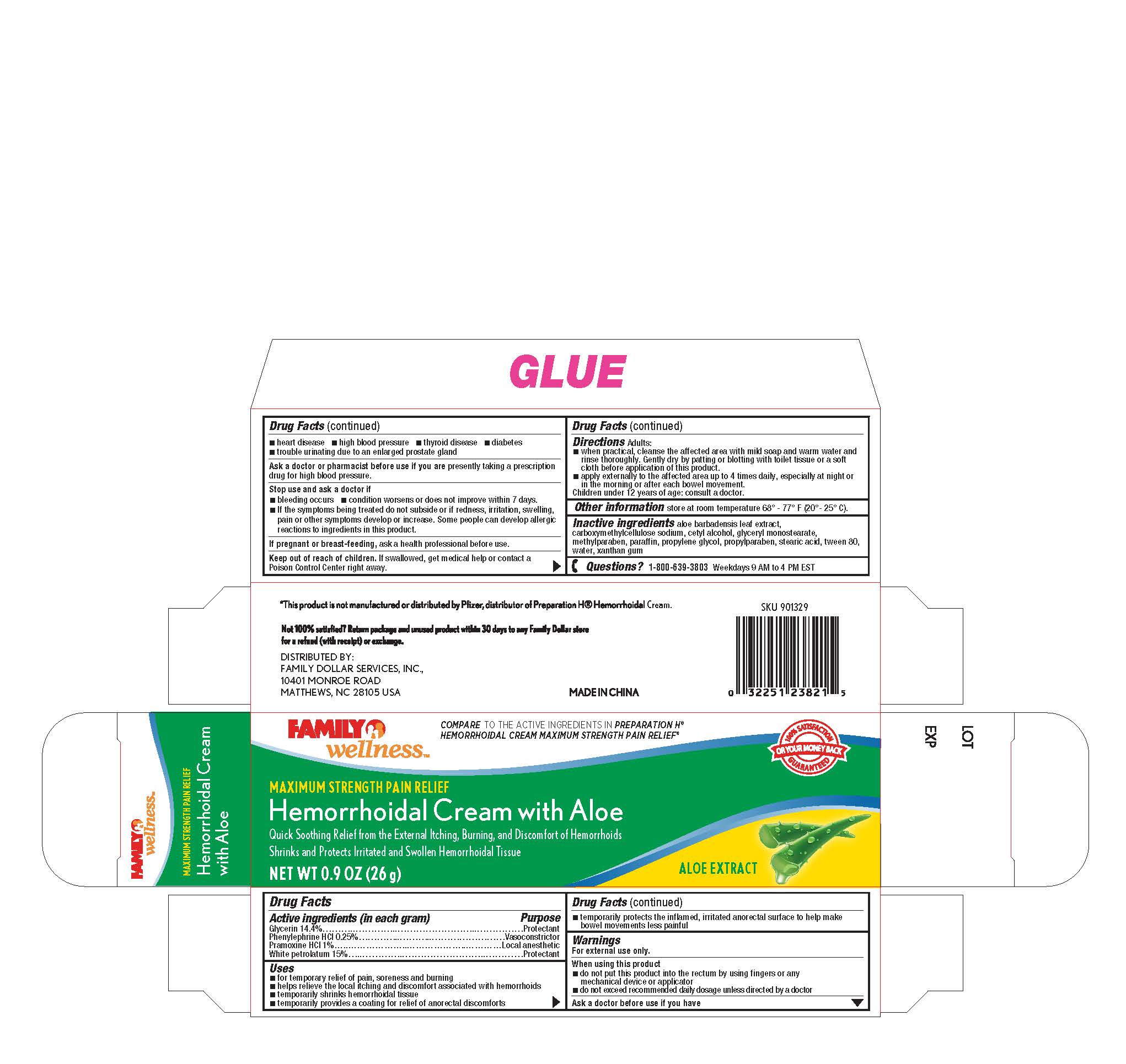
Label: FAMILY WELLNESS- glycerin, phenylephrine hcl, pramoxine hcl, petrolatum cream
- NDC Code(s): 69571-004-01, 69571-004-02
- Packager: FRONT PHARMACEUTICAL PLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
- for temporary relief of pain, soreness and burning
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporarily shrinks hemorrhoidal tissue
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
-
WARNINGS
Warnings
. For external use only
When using this product
Ask a doctor before use if you have
•heart disease •high blood pressure •thyroid disease •diabetes
•trouble urinating due to an enlarged prostate gland
you are presently taking a prescription Ask a doctor or pharmacist before use if
drug for high blood pressure.
Stop use and ask a doctor if
•bleeding occurs •condition worsens or does not improve within 7 days
-if the symptoms being treated do not subside or ir redness, irritation, swelling,
pain or other symptoms develop or increase. Some people can develop allergic
reactions to ingredients in this product.
, ask a health professional before use. If pregnant or breast-feeding
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- do not exceed recommended daily dosage unless directed by a doctor
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults: Directions
-when practical, cleanse the affected area with mild soap and warm water and
rinse thoroughly. Gentlydry by patting or blotting with toilet tissue or a soft
cloth before application of this product.
-apply externally to the affected area up to 4 times daily especially at night r
in the morningor after each bowel movement.
hildren under 12 years of age: consult a doctor.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FAMILY WELLNESS
glycerin, phenylephrine hcl, pramoxine hcl, petrolatum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69571-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 14.4 g in 100 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 0.25 g in 100 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 1 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 15 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PARAFFIN (UNII: I9O0E3H2ZE) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) STEARIC ACID (UNII: 4ELV7Z65AP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69571-004-02 1 in 1 BOX 03/14/2017 1 NDC:69571-004-01 26 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/14/2017 Labeler - FRONT PHARMACEUTICAL PLC (530897792) Establishment Name Address ID/FEI Business Operations FRONT PHARMACEUTICAL PLC 530897792 manufacture(69571-004)