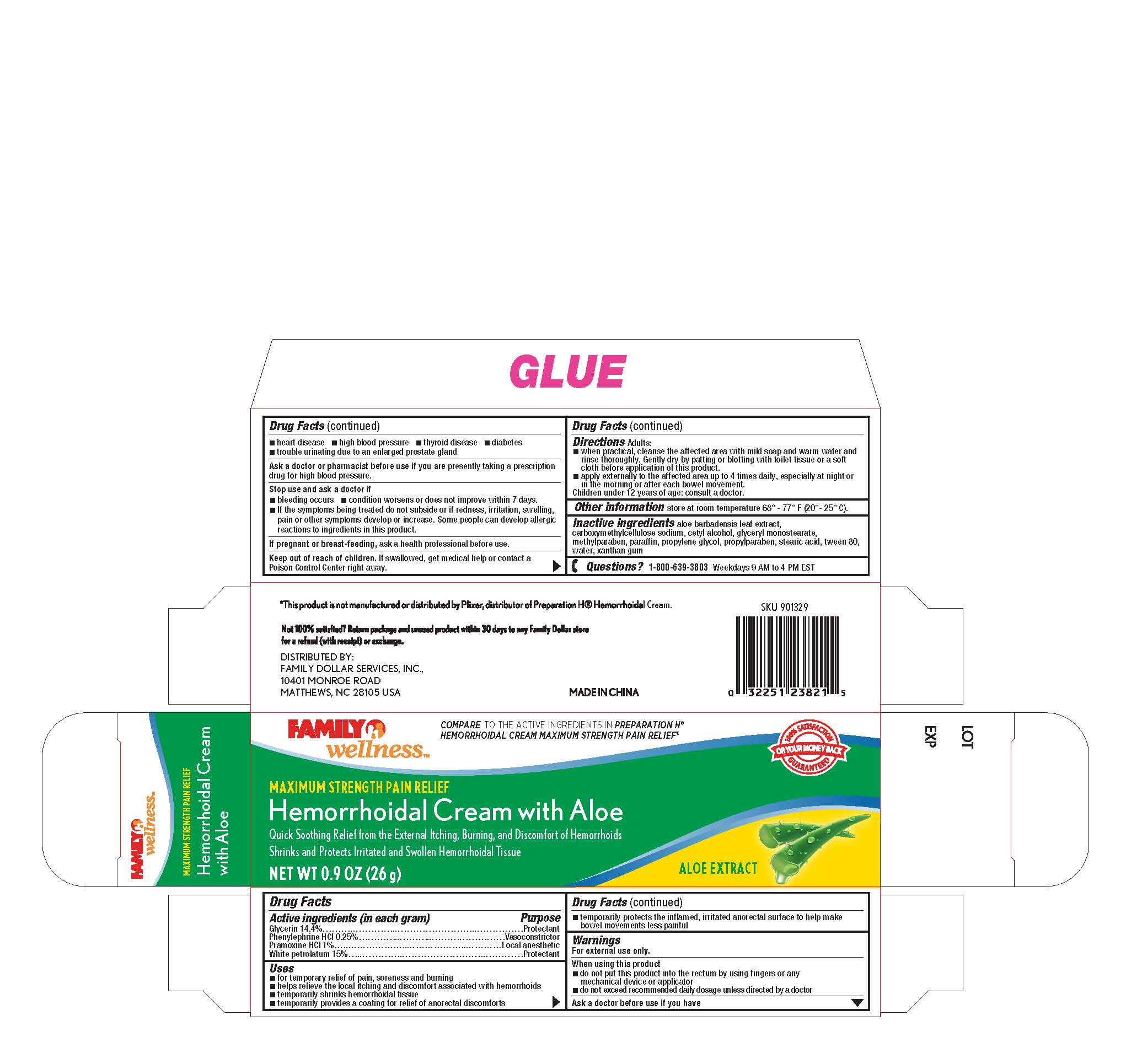
Active ingredients (in each gram)
Glycerin 14.4%
Phenylephrine HCL 0.25%
Pramoxine HCL 1%
White petrolatum 15%
Uses
- for temporary relief of pain, soreness and burning
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporarily shrinks hemorrhoidal tissue
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
Warnings
. For external use only
When using this product
Ask a doctor before use if you have
•heart disease •high blood pressure •thyroid disease •diabetes
•trouble urinating due to an enlarged prostate gland
you are presently taking a prescription Ask a doctor or pharmacist before use if
drug for high blood pressure.
Stop use and ask a doctor if
•bleeding occurs •condition worsens or does not improve within 7 days
-if the symptoms being treated do not subside or ir redness, irritation, swelling,
pain or other symptoms develop or increase. Some people can develop allergic
reactions to ingredients in this product.
, ask a health professional before use. If pregnant or breast-feeding
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- do not exceed recommended daily dosage unless directed by a doctor
. If swallowed, get medical help or contact a Keep out of reach of children
Poison Control Center right away.
Adults: Directions
-when practical, cleanse the affected area with mild soap and warm water and
rinse thoroughly. Gentlydry by patting or blotting with toilet tissue or a soft
cloth before application of this product.
-apply externally to the affected area up to 4 times daily especially at night r
in the morningor after each bowel movement.
hildren under 12 years of age: consult a doctor.