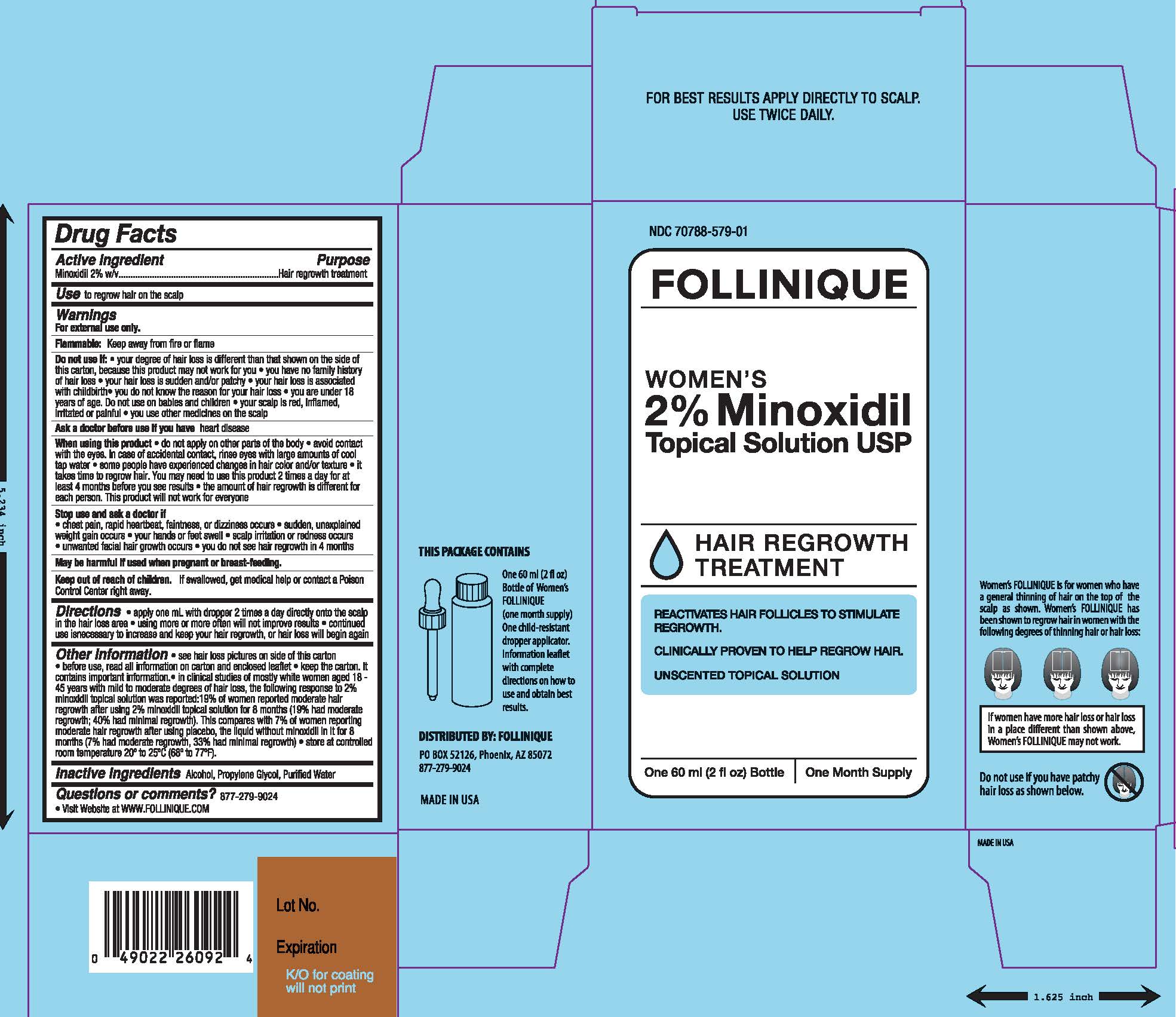
Label: FOLLINIQUE- minoxidil solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 70788-579-01 - Packager: Premium Distribution, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 27, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients:
- Purpose
- Use
- Warnings
-
Do not use if
- your degree of hair loss is different than that shown on the side of this carton, because this product may not work for you
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- your hair loss is associated with childbirth
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medications on the scalp
- ASK DOCTOR
-
When using this product
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap waler.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. You may need to use this product 2 times a day at least 4 months before you see results
- the amount of hair regrowth is different for each person. This product will not work for everyone
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Other Information
- see hair loss pictures on side of this carton
- before use, read all information on carton and enclosed leaflet
- in clinical research in mostly white women age 18-45 years with mild to moderate degrees of hair loss, the following response to Minoxidil Topical Solution 2% was reported: 19% of women reported moderate hair regrowth after using women's Minoxidil Topical Solution 2% for 8 months; 19% had moderate regrowth; 40% had minimal regrowth. This compares with 7% of women reporting hair regrowth after using the placebo, the liquid without Minoxidil in it, for 8 months (7% had moderate regrowth, 33% had minimal regrowth)
- keep the carton. It contains important information.
- store at controlled room temperature 200 to 250C (680 to 770F).
- Inactive Ingredients:
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOLLINIQUE
minoxidil solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70788-579 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70788-579-01 1 in 1 CARTON 10/27/2016 1 60 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078176 10/27/2016 Labeler - Premium Distribution, LLC (079805390)