Label: BIO-SCRIPTIVES EXTREME AF- tolnaftate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 60608-013-00 - Packager: BioChemics, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 27, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
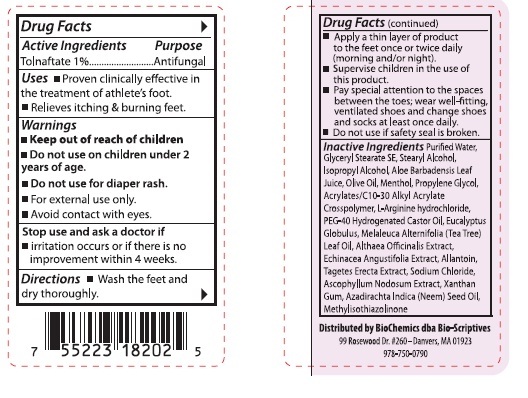
- Active Ingredients
- Purpose
- Uses
- Warnings
- Stop use and ask a doctor if
-
Directions
■ Wash the feet and dry thoroughly.
■ Apply a thin layer of product to the feet once or twice daily (morning and/or night).
■ Supervise children in the use of this product.
■ Pay special attention to the spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily.
■ Do not use if safety seal is broken.
-
Inactive Ingredients
Purified Water, Glyceryl Stearate SE, Stearyl Alcohol, Isopropyl Alcohol, Aloe Barbadensis Leaf Juice, Olive Oil, Menthol, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, L-Arginine hydroxide, PEG-40 Hdrogenated Castor Oil, Eucalyptus Globulus, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Althaea Officinalis Extract, Echinacea Angustifolia Extract, Allantoin, Tagetes Erecta Extract, Sodium Chloride, Ascophyllum Nodosum Extract, Xanthan Gum, Azadirachta Indica (Neem) Seed Oil, Methylisothiazolinone
- Reference
- Labeling
- Keep out of reach of children
-
INGREDIENTS AND APPEARANCE
BIO-SCRIPTIVES EXTREME AF
tolnaftate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60608-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE .56 g in 56.69 g Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) ECHINACEA ANGUSTIFOLIA LEAF (UNII: FS7G8S6PJ8) ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) TAGETES ERECTA FLOWER (UNII: UH5X33P33E) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) MENTHOL (UNII: L7T10EIP3A) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OLIVE OIL (UNII: 6UYK2W1W1E) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) AZADIRACHTA INDICA SEED OIL (UNII: 4DKJ9B3K2T) ISOPROPYL ALCOHOL (UNII: ND2M416302) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60608-013-00 56.69 g in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 07/27/2011 Labeler - BioChemics, Inc. (802946426) Establishment Name Address ID/FEI Business Operations Coastal Products Company 782445688 manufacture