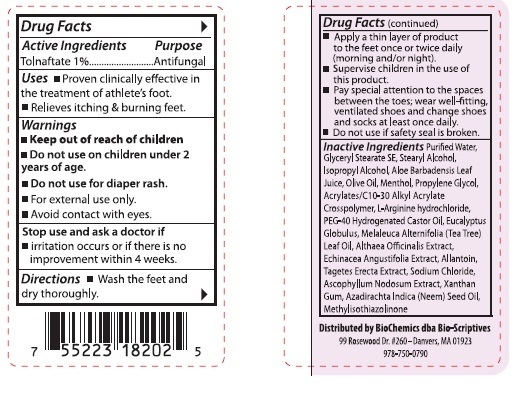
Uses
■ Proven clinically effective in the treatment of athlete's foot.■ Relieves itching and burning feet.
Warnings
■ Do not use on children under 2 years of age.■ Do not use for diaper rash.
■ For external use only.
■ Avoid contact with eyes.
Directions
■ Wash the feet and dry thoroughly.■ Apply a thin layer of product to the feet once or twice daily (morning and/or night).
■ Supervise children in the use of this product.
■ Pay special attention to the spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily.
■ Do not use if safety seal is broken.
Inactive Ingredients
Purified Water, Glyceryl Stearate SE, Stearyl Alcohol, Isopropyl Alcohol, Aloe Barbadensis Leaf Juice, Olive Oil, Menthol, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, L-Arginine hydroxide, PEG-40 Hdrogenated Castor Oil, Eucalyptus Globulus, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Althaea Officinalis Extract, Echinacea Angustifolia Extract, Allantoin, Tagetes Erecta Extract, Sodium Chloride, Ascophyllum Nodosum Extract, Xanthan Gum, Azadirachta Indica (Neem) Seed Oil, Methylisothiazolinone