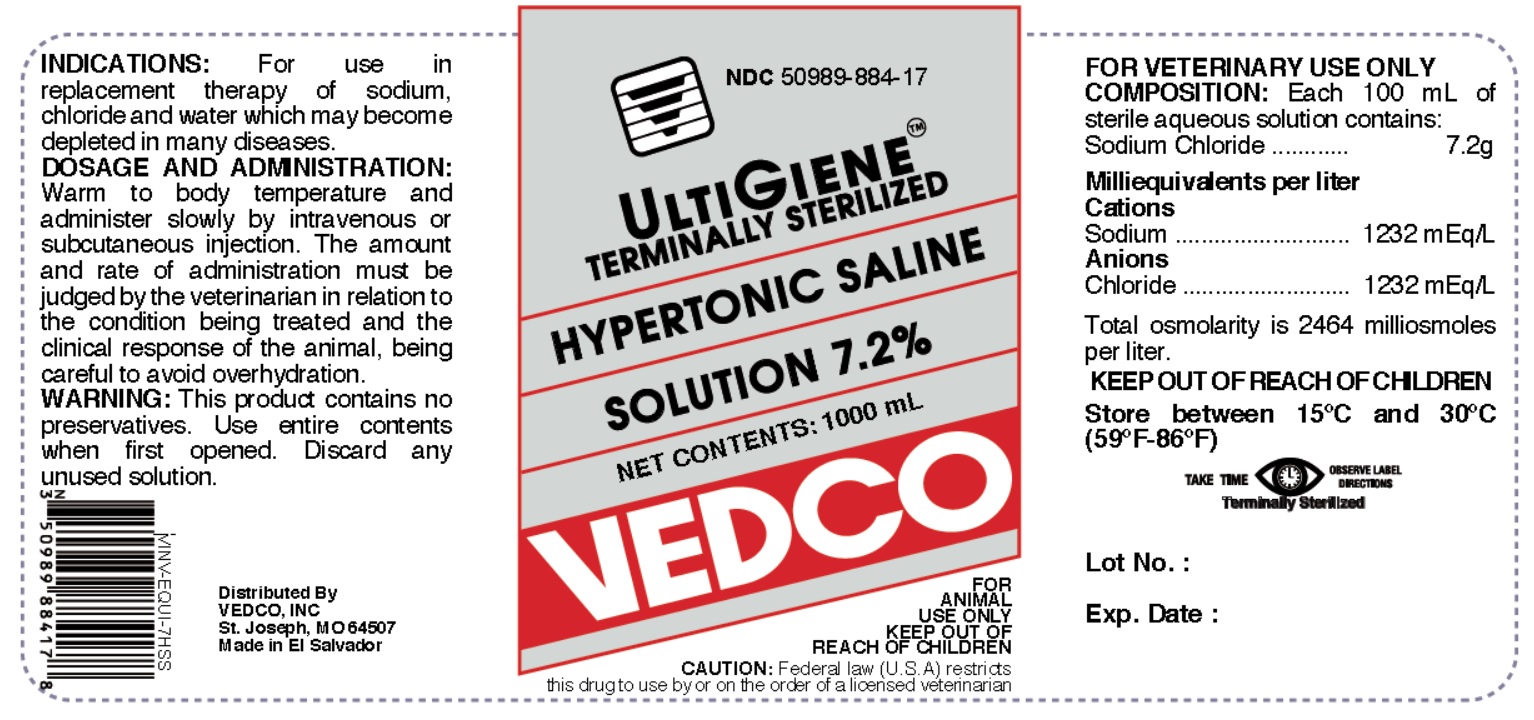
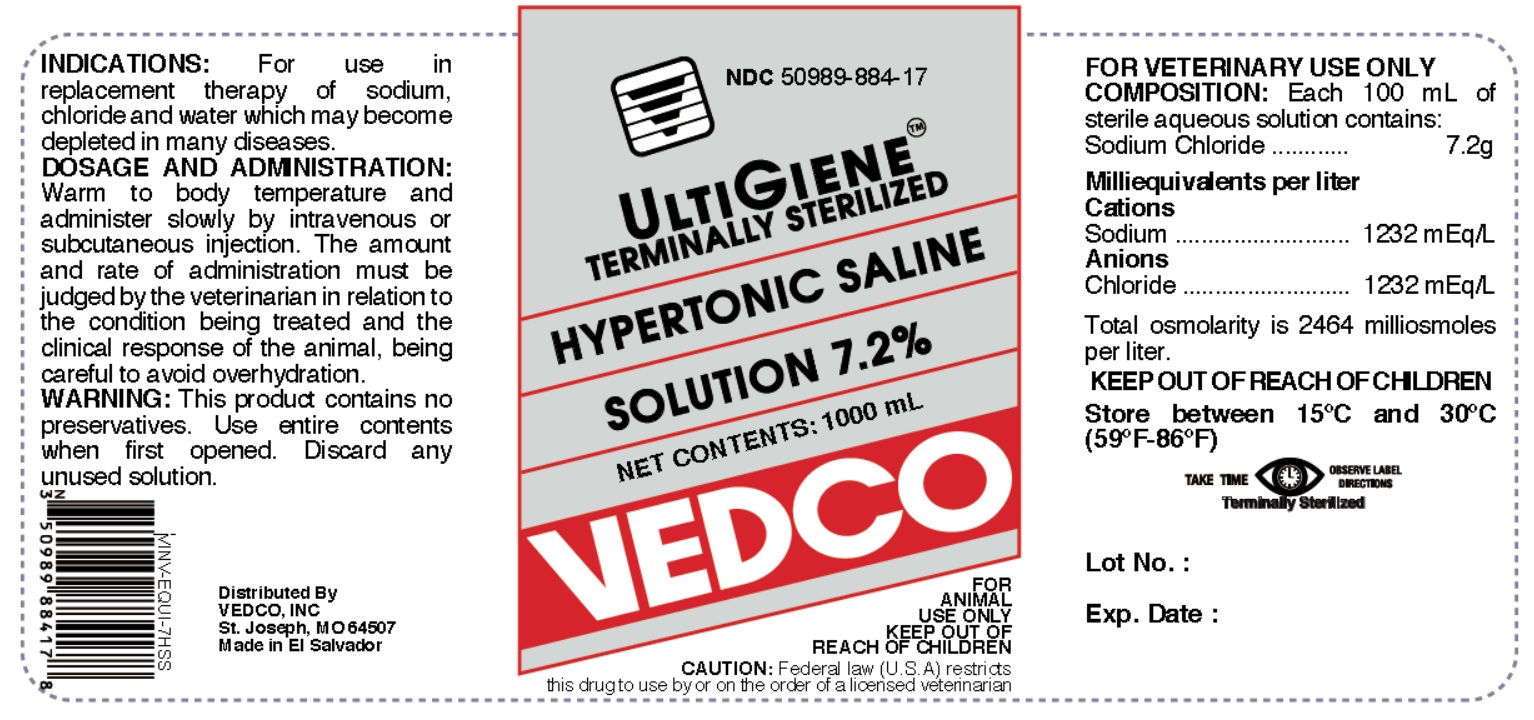
Label: HYPERTONIC SALINE 7.2 % injection, solution
- NDC Code(s): 50989-884-17
- Packager: Vedco
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 15, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
ACTIVE INGREDIENT
Each 100 mL of sterile aqueous solution contains:
Sodium Chloride.................................................7.2 gMilliequivalents per liter:
Cations Sodium.......................................1232 mEq/L
Anions Chloride......................................1232 mEq/LTotal osmolarity is 2464 milliosmoles per litter.
- INDICATION
-
DOSAGE AND AND ADMINISTRATION
50 to 100 mL per lb bodyweight. Warm to body temperature and administer slowly by intravenous or subcutaneous injection. The amount and rate of administration must be judged by the veterinarian in relation to the condition being treated and the clinical response of the animal, being careful to avoid overhydration.
- CAUTION
- STORAGE
- INFORMATION FOR OWNERS/CAREGIVERS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYPERTONIC SALINE 7.2 %
hypertonic saline 7.2 % injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:50989-884 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 7.2 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50989-884-17 1000 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/09/2019 Labeler - Vedco (021634266) Registrant - Vedco (021634266) Establishment Name Address ID/FEI Business Operations Laboratorios Biogalenic 851259507 manufacture Establishment Name Address ID/FEI Business Operations K+S Minerals and Agriculture GmbH 507531213 api manufacture