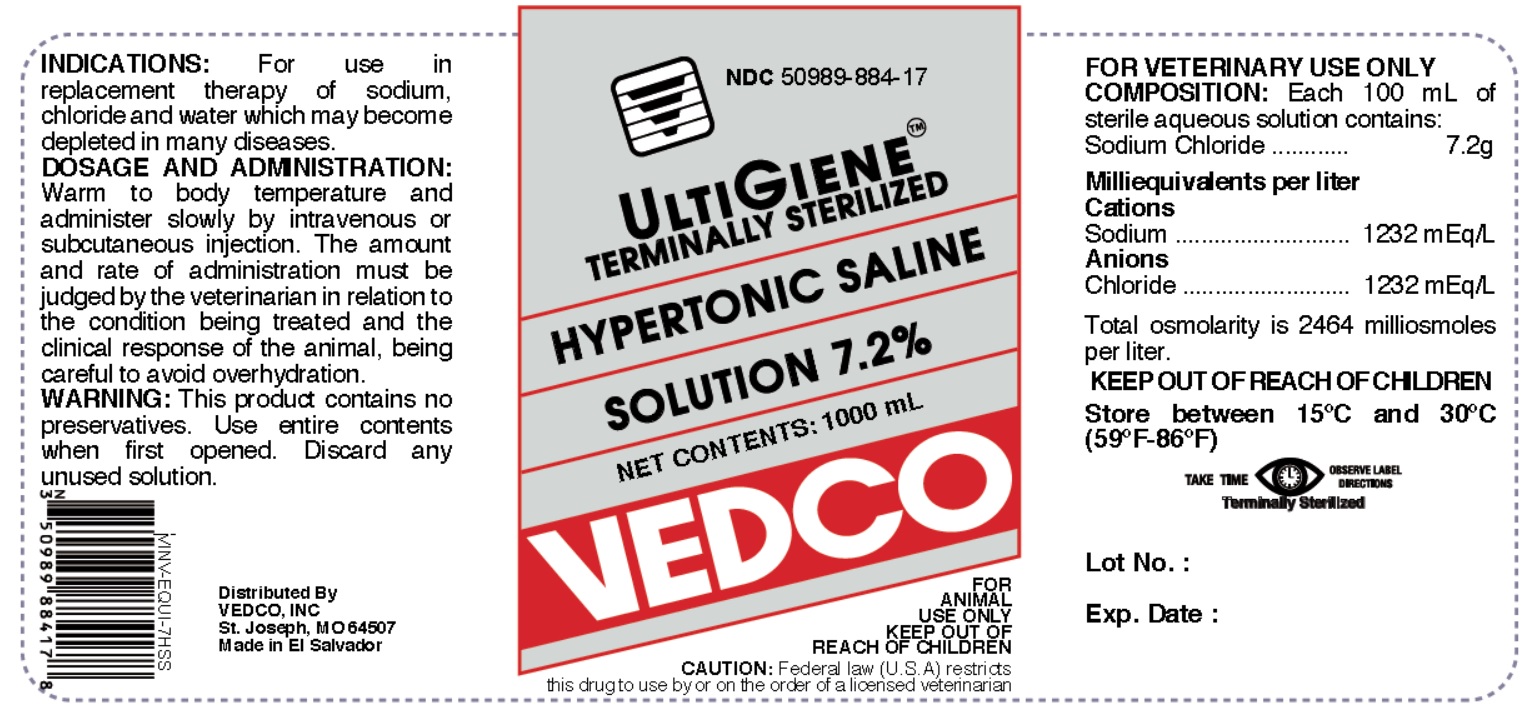
HYPERTONIC SALINE 7.2 %- hypertonic saline 7.2 % injection, solution
Vedco
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
Hypertonic Saline Solution 7.2%
Sterile - Preservative Free
KEEP OUT OF REACH OF CHILDREN
ACTIVE INGREDIENT
Each 100 mL of sterile aqueous solution contains:
Sodium Chloride.................................................7.2 g
Milliequivalents per liter:
Cations Sodium.......................................1232 mEq/L
Anions Chloride......................................1232 mEq/L
Total osmolarity is 2464 milliosmoles per litter.
INDICATION
For use in replacement therapy of sodium, chloride and water which may become depleted in many diseases.
DOSAGE AND AND ADMINISTRATION
50 to 100 mL per lb bodyweight. Warm to body temperature and administer slowly by intravenous or subcutaneous injection. The amount and rate of administration must be judged by the veterinarian in relation to the condition being treated and the clinical response of the animal, being careful to avoid overhydration.
CAUTION
This product contains no preservatives. Do not use if solution is not clear. Use entire contents when first opened. Discard any unused solution.
Federal law restricts this drug to use by or on the order of a licensed veterinarian.