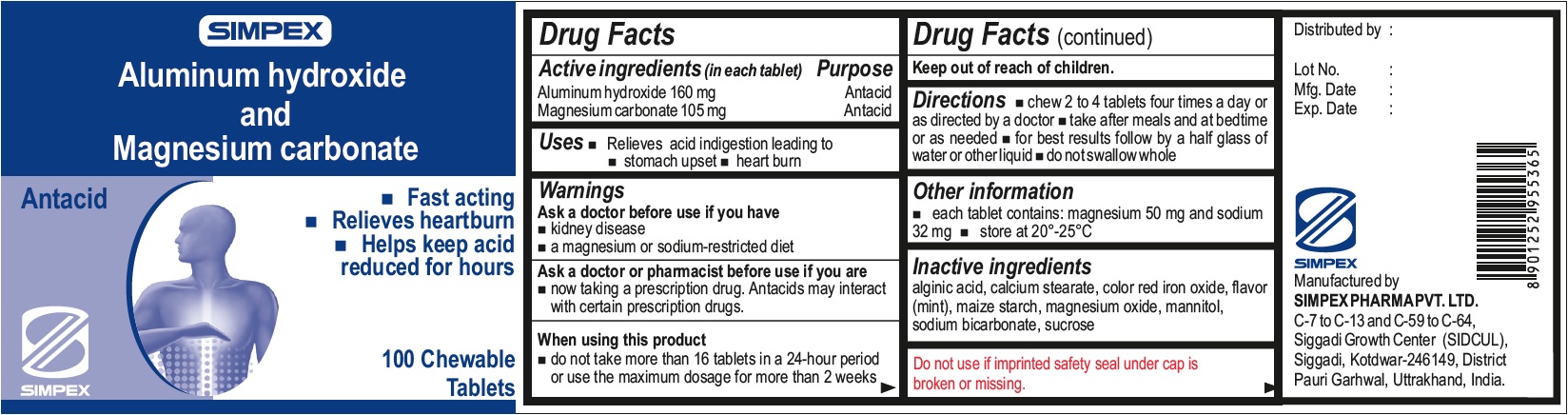
Label: ALUMINUM HYDROXIDE AND MAGNESIUM CARBONATE- aluminum hydroxide, magnesium carbonate tablet, chewable
- NDC Code(s): 76457-001-00
- Packager: Simpex Pharma Pvt. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ALUMINUM HYDROXIDE AND MAGNESIUM CARBONATE
aluminum hydroxide, magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76457-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYL SALICYLATE (UNII: LAV5U5022Y) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) MANNITOL (UNII: 3OWL53L36A) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SUCROSE (UNII: C151H8M554) Product Characteristics Color orange (orange) Score 2 pieces Shape ROUND (round biconvex film-coated tablet plain from both sides) Size 13mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76457-001-00 1 in 1 CARTON 02/09/2018 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 02/09/2018 Labeler - Simpex Pharma Pvt. Ltd (916758275) Registrant - Simpex Pharma Pvt. Ltd (916758275) Establishment Name Address ID/FEI Business Operations Simpex Pharma Pvt. Ltd 916758275 manufacture(76457-001)