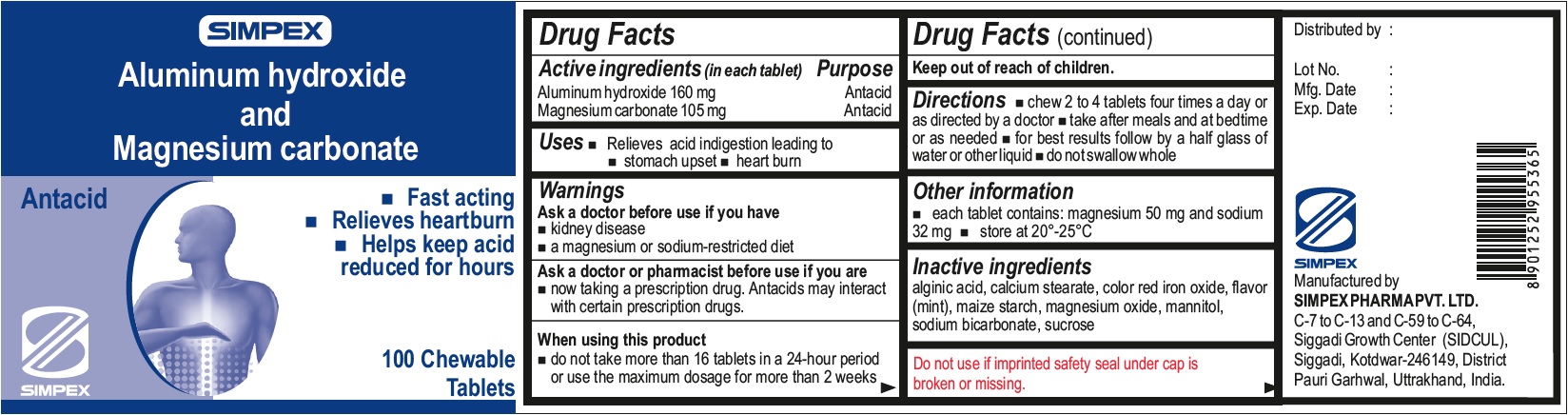
ALUMINUM HYDROXIDE AND MAGNESIUM CARBONATE
- aluminum hydroxide, magnesium carbonate tablet, chewable
Simpex Pharma Pvt. Ltd
----------
Active ingredient (in each tablet)
Aluminum hydroxide, 160 mg
Magnesium carbonate, 105 mg
Uses
Relieves acid indigestion leading to
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium or sodium-restricted diet
Ask a doctor or pharmacist before use if you are
- now taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
do not take more than 16 tablets in a 24-hour period or use the maximum dosage for more than 2 weeks
Keep out of reach of children.
Directions
- chew 2 to 4 tablets four times a day or as directed by a doctor
- take after meals and at bedtime or as needed
- for best results follow by a half glass of water or other liquid
- do not swallow whole
Other information
- each tablet contains: magnesium 50 mg and sodium 32 mg
- store at 20°-25°C
Inactive ingredients
alginic acid, calcium stearate, color red iron oxide, flavor (mint), maize starch, magnesium oxide, mannitol, sodium bicarbonate, sucrose
Package Labeling:
Simpex Pharma Pvt. Ltd
