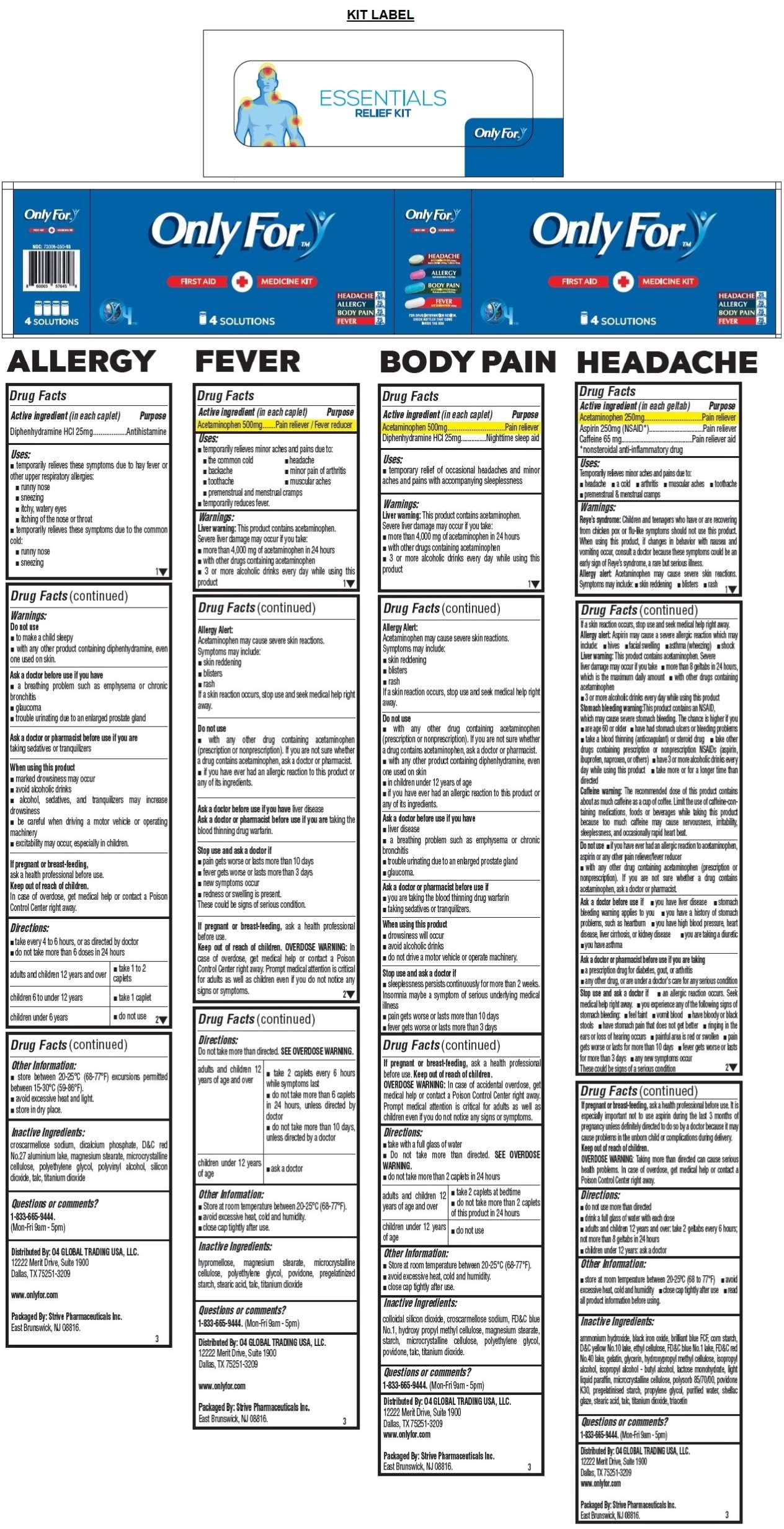
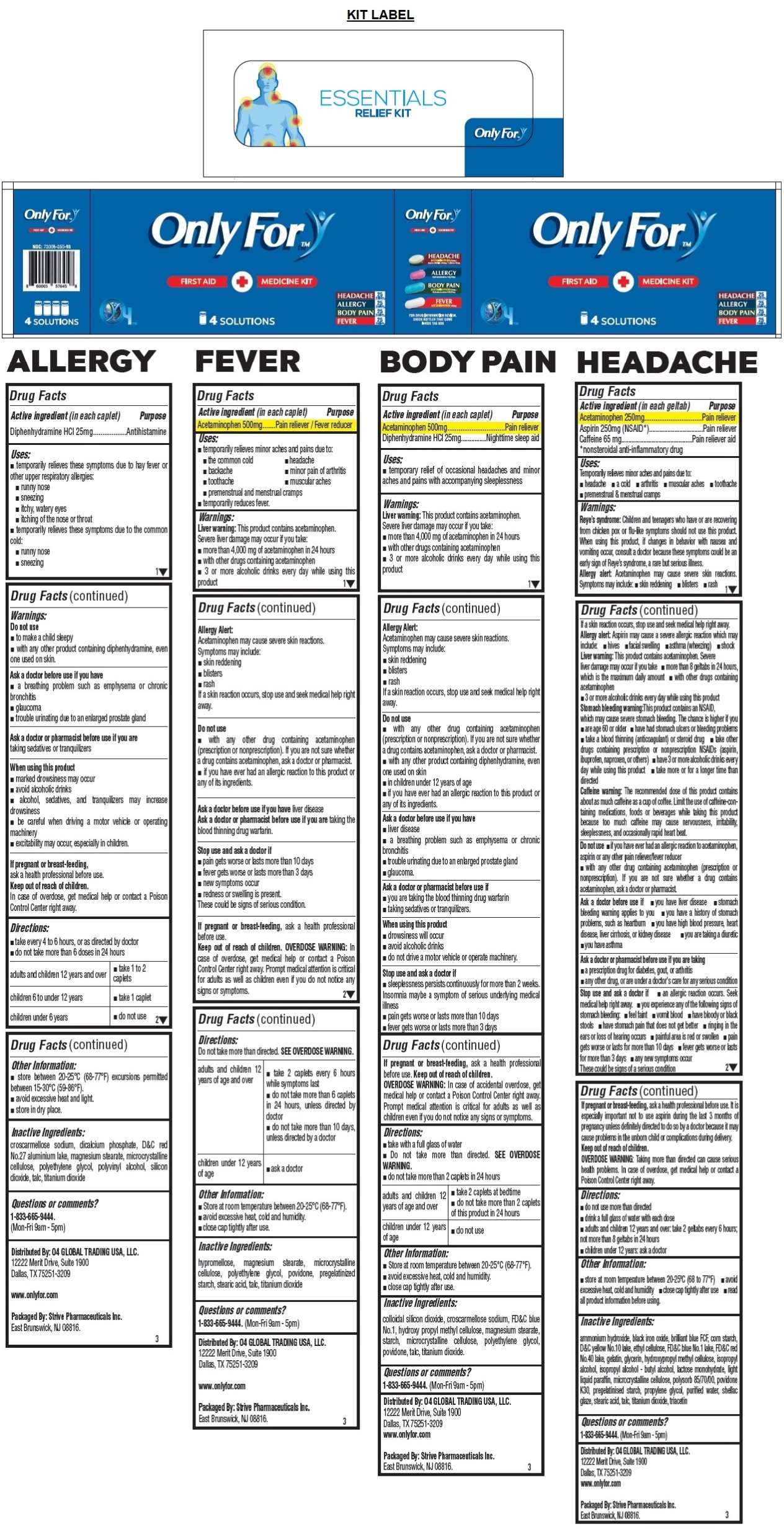
Label: ONLY FOR FIRST AID MEDICINE- diphenhydramine hcl, acetaminophen, aspirin, caffeine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 73006-120-95, 73006-132-95, 73006-143-95, 73006-148-95, view more73006-350-98 - Packager: O4 Global Trading Usa, Llc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ONLY FOR HEADACHE- acetaminophen, aspirin, caffeine tablet
- Active ingredient (in each geltab)
- Purpose
- Uses:
-
Warnings:
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flue-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include: • skin reddening • blisters • rash
If a skin reaction occurs, stop use and seek medical help right away.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: • hives • facial swelling • asthma (wheezing) • shock
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take • more than 8 geltabs in 24 hours, which is the maximum daily amount • with other drugs containing acetaminophen • 3 or more alcoholic drinks every day while using this product
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you • are age 60 or older • have had stomach ulcers or bleeding problems • take a blood thinning (anticoagulant) or steroid drug • take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, or others) • have 3 or more alcoholic drinks every day while using this product • take more or for a longer time than directed
Caffeine warning: The recommended dose of this product contains about a much caffeine as cup of coffee. Limit the use of caffeine-containing medications, foods or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally rapid heart beat.
Do not use • if you have ever had an allergic reaction to acetaminophen, aspirin or any other pain reliever/fever reducer • with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if • you have liver disease • stomach bleeding warning applies to you • you have a history of stomach problems, such as heartburn • you have high blood pressure, heart disease, liver cirrhosis, or kidney disease • you are taking a diuretic • you have asthma
Ask a doctor or pharmacist before use if you are taking
• a prescription drug for diabetes, gout, or arthritis
• any other drug, or are under a doctor's care for any serious condition
Stop use and ask a doctor if • an allergic reaction occurs. Seek medical help right away. • you experience any of the following signs of stomach bleeding: • feel faint • vomit blood • have bloody or black stools • have stomach pain that does not get better • ringing in the ears or loss of hearing occurs • painful area is red or swollen • pain gets worse or lasts for more than 10 days • fever gets worse or lasts for more than 3 days • any new symptoms occur
These could be signs of a serious condition
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or compliments during delivery.
- Directions:
- Other Information:
-
Inactive Ingredients:
ammonium hydroxide, black iron oxide, brilliant blue FCF, corn starch, D&C yellow No.10 lake, ethyl cellulose, FD&C blue No.1 lake, FD&C red No.40 lake, gelatin, glycerin, hydroxypropyl methyl cellulose, isopropyl alcohol, isopropyl alcohol - butyl alcohol, lactose monohydrate, light liquid paraffin, microcrystalline cellulose, polysorb 85/70/00, povidone K30, pregelatinised starch, propylene glycol, purified water, shellac glaze, stearic acid, talc, titanium dioxide, triacetin
- Questions or comments?
- ONLY FOR ALLERGY- diphenhydramine hcl tablet
- Active ingredient (in each caplet)
- Purpose
- Uses:
-
Warnings:
Do not use
• to make a child sleepy
• with any other product containing diphenhydramine, even one used on skin.Ask a doctor before use if you have
• a breathing problem such as emphysema or chronic bronchitis
• glaucoma
• trouble urinating due to an enlarged prostate glandAsk a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
• marked drowsiness may occur
• avoid alcoholic drinks
• alcohol, sedatives, and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children.If pregnant or breast-feeding,
ask a health professional before use. - Directions:
- Other Information:
- Inactive Ingredients:
- Questions or comments?
- ONLY FOR BODY PAIN- acetaminophen, diphenhydramine hcl tablet
- Active ingredient (in each caplet)
- Purpose
- Uses:
-
Warnings:
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
• more than 4,000 mg of acetaminophen in 24 hours
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Allergy Alert:
Acetaminophen may cause severe skin reactions.
Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• with any other product containing diphenhydramine, even one used on skin
• in children under 12 years of age
• if you have ever had any allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
• liver disease
• a breathing problem such as emphysema or chronic bronchitis
• trouble urinating due to an enlarged prostate gland
• glaucoma.
Ask a doctor or pharmacist before use if
• you are taking the blood thinning drug warfarin
• taking sedatives or tranquilizers.
When using this product
• drowsiness will occur
• avoid alcoholic drinks
• do not drive a motor vehicle or operate machinery.
Stop use and ask a doctor if
• sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
• pain gets worse or lasts more than 10 days
• fever gets worse or last more than 3 days
If pregnant or breast-feeding, ask a health professional before use.
-
Directions:
• take with a full glass of water
• Do not take more than directed. SEE OVERDOSE WARNING.
• do not take more than 2 caplets in 24 hours
adults and children 12 years of age and over • take 2 caplets at bedtime • do not take more than 2 caplets of this product in 24 hours
children under 12 years of age • do not use - Other Information:
- Inactive Ingredients:
- Questions or comments?
- ONLY FOR FEVER- acetaminophen tablet
- Active ingredient (in each caplet)
- Purpose
- Uses:
-
Warnings:
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
• more than 4,000 mg of acetaminophen in 24 hours
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Allergy Alert:
Acetaminophen may cause severe skin reactions.
Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you have ever had any allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have liver disease
Ask a doctor or pharmacist before use if are taking the blood thinning drug warfarin
Stop use and ask a doctor if
• pain gets worse or lasts more than 10 days
• fever gets worse or last more than 3 days
• new symptoms occur.
• redness or swelling is present
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions:
Do not take more than directed. SEE OVERDOSE WARNING.
adults and children 12 years of age and over • take 2 caplets every 6 hours while symptoms last • do not take more than 6 caplets in 24 hours, unless directed by doctor
• do not take more than 10 days, unless directed by a doctor
children under 12 years of age • ask a doctor - Other Information:
- Inactive Ingredients:
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Only For™ FIRST AID + MEDICINE KIT
4 SOLUTIONS
HEADACHE 25 Geltabs
ACETAMINOPHEN 250mg / Aspirin (NSAID) 250mg / Caffeine 65mg
ALLERGY 25 Caplets
Diphenhydramine HCl 25mg
BODY PAIN 25 Caplets
ACETAMINOPHEN 500mg / Diphenhydramine HCl 25mg
FEVER 25 Caplets
ACETAMINOPHEN 500mg
FOR DRUG INFORMATION REVIEW, CHECK BOTTLES THAT COME INSIDE THE BOX
Distributed By: O4 GLOBAL TRADING USA, LLC.12222 Merit Drive, Suite 1900
Dallas, TX 75251-3209
www.onlyfor.com
Packaged By: Strive Pharmaceuticals Inc.
East Brunswick, NJ 08816.
- Packaging
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONLY FOR FIRST AID MEDICINE
diphenhydramine hcl, acetaminophen, aspirin, caffeine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73006-350 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73006-350-98 1 in 1 BOX; Type 0: Not a Combination Product 11/15/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 25 Part 2 1 BOTTLE, PLASTIC 25 Part 3 1 BOTTLE, PLASTIC 25 Part 4 1 BOTTLE, PLASTIC 25 Part 1 of 4 ONLY FOR HEADACHE
acetaminophen, aspirin, caffeine tabletProduct Information Item Code (Source) NDC:73006-132 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) LIGHT MINERAL OIL (UNII: N6K5787QVP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL SOLUTION (UNII: 8KW3E207O2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color green (Dark Green - White) Score no score Shape ROUND Size 13mm Flavor Imprint Code S77 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73006-132-95 1 in 1 CARTON 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 11/15/2020 Part 2 of 4 ONLY FOR ALLERGY
diphenhydramine hcl tabletProduct Information Item Code (Source) NDC:73006-148 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL (Caplet) Size 11mm Flavor Imprint Code DP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73006-148-95 1 in 1 CARTON 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part338 11/15/2020 Part 3 of 4 ONLY FOR BODY PAIN
acetaminophen, diphenhydramine hcl tabletProduct Information Item Code (Source) NDC:73006-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue (LIGHT BLUE) Score no score Shape capsule Size 18mm Flavor Imprint Code AD01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73006-120-95 1 in 1 CARTON 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 11/15/2020 Part 4 of 4 ONLY FOR FEVER
acetaminophen tabletProduct Information Item Code (Source) NDC:73006-143 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape capsule Size 17mm Flavor Imprint Code S500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73006-143-95 1 in 1 CARTON 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 11/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part338 11/15/2020 Labeler - O4 Global Trading Usa, Llc (081226861)