Label: BACITRACIN ZINC AND POLYMYXIN B SULFATE ointment
- NDC Code(s): 68788-7599-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 24208-555
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Bacitracin zinc and polymyxin B sulfate ophthalmic ointment, USP is a sterile antimicrobial ointment formulated for ophthalmic use.
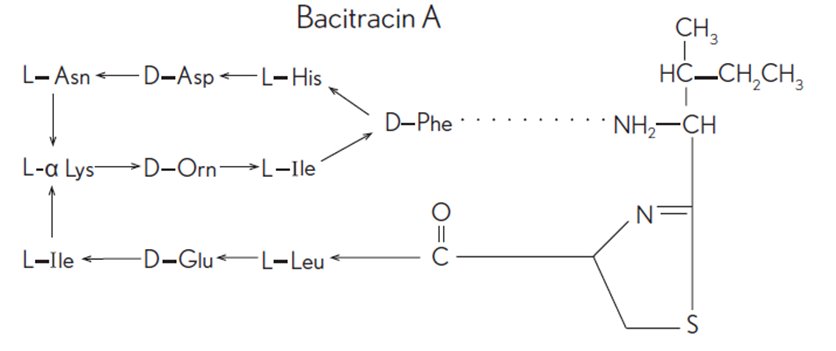
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the licheniformis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units/mg. The structural formula for bacitracin A is:
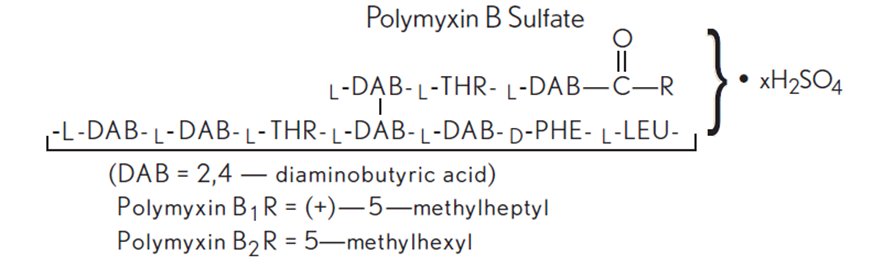
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units/mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Actives: bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units; Inactives: mineral oil and white petrolatum.
- CLINICAL PHARMACOLOGY
- INDlCATlONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Bacitracin zinc and polymyxin B sulfate ophthalmic ointment, USP is available in tubes with an ophthalmic tip applicator in the following size:
NDC 68788-7599-3 - 3.5 g tube
Storage:
Store between 15°C to 25°C (59°F to 77°F). KEEP TIGHTLY CLOSED.
KEEP OUT OF REACH OF CHILDREN.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USAManufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA© 2022 Bausch & Lomb Incorporated or its affiliates
Relabeled By: Preferred Pharmaceuticals Inc.
Revised: May 2022
9130704 (Folded)
9130604 (Flat) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACITRACIN ZINC AND POLYMYXIN B SULFATE
bacitracin zinc and polymyxin b sulfate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68788-7599(NDC:24208-555) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7599-3 1 in 1 CARTON 03/27/2020 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064046 03/27/2020 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-7599)