Label: MAGNELEAF CBD THC- menthol, arnica montana, methyl salicylate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 82900-112-13 - Packager: M J Green Enterprises, INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 22, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
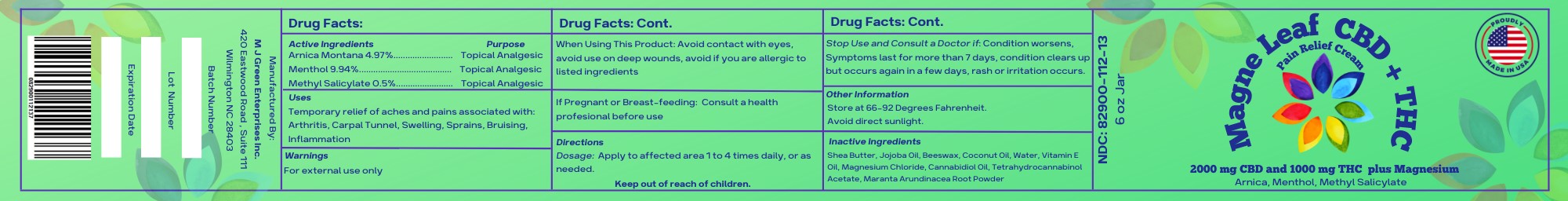
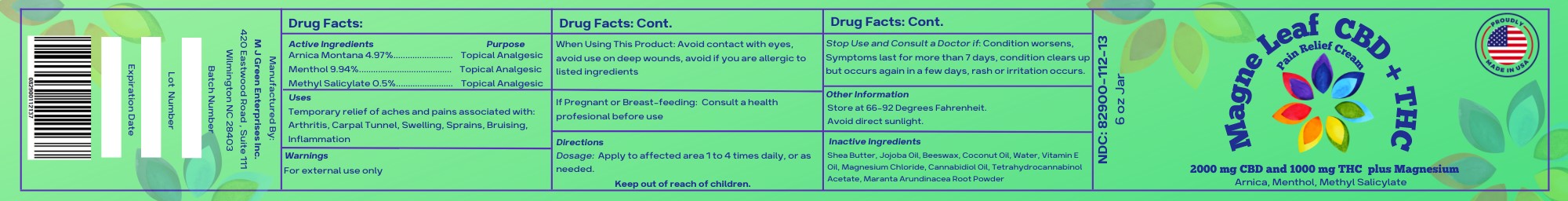
- Active Ingredients
- Uses
- Warnings
- When Using This Product
- If Pregnant of Breast-Feeding:
- Directions
- Other Information
- Inactive Ingredients
- 6 ounce jar label
- 6 ounce lid label
-
INGREDIENTS AND APPEARANCE
MAGNELEAF CBD THC
menthol, arnica montana, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82900-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 8.79 g in 170.96 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 17 g in 170.96 g CANNABIDIOL (UNII: 19GBJ60SN5) (CANNABIDIOL - UNII:19GBJ60SN5) CANNABIDIOL 2 g in 170.96 g MARANTA ARUNDINACEA ROOT (UNII: FVN346W31A) (MARANTA ARUNDINACEA ROOT - UNII:FVN346W31A) MARANTA ARUNDINACEA ROOT 1 g in 170.96 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 8.5 g in 170.96 g ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) (ALPHA-TOCOPHEROL - UNII:H4N855PNZ1) ALPHA-TOCOPHEROL 6.52 g in 170.96 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 1 g in 170.96 g Inactive Ingredients Ingredient Name Strength MAGNESIUM CHLORIDE (UNII: 02F3473H9O) 4.25 g in 170.96 g COCONUT OIL (UNII: Q9L0O73W7L) 12.4 g in 170.96 g PEG-8 BEESWAX (UNII: 3C1QUF1TIR) 12.4 g in 170.96 g SHEA BUTTER (UNII: K49155WL9Y) 48.05 g in 170.96 g JOJOBA OIL (UNII: 724GKU717M) 48.05 g in 170.96 g .DELTA.9-TETRAHYDROCANNABINOL ACETATE (UNII: VHV54M2AES) 1 g in 170.96 g Product Characteristics Color white (Cream) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82900-112-13 170.96 g in 1 JAR; Type 0: Not a Combination Product 11/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/22/2022 Labeler - M J Green Enterprises, INC. (099497973) Establishment Name Address ID/FEI Business Operations M J Green Enterprises, INC. 099497973 manufacture(82900-112)


 MagneLeaf THC Pain Relief Lid Label
MagneLeaf THC Pain Relief Lid Label