MAGNELEAF CBD THC- menthol, arnica montana, methyl salicylate cream
M J Green Enterprises, INC.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
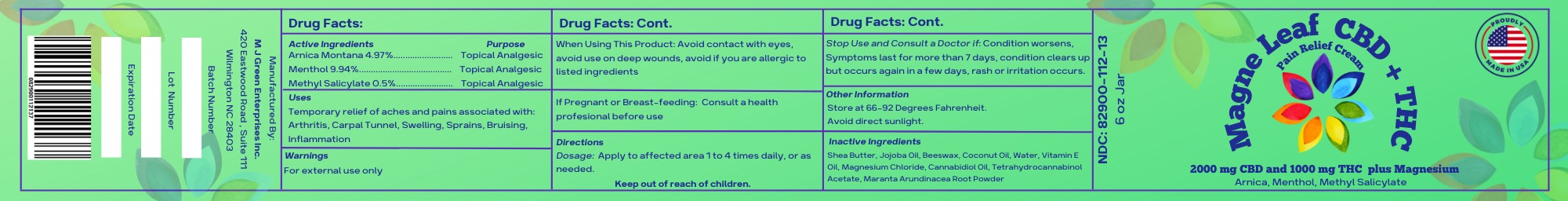
Active Ingredients
Menthol 9.94%
Arnica Montana 4.97%
Methyl Salicylate 0.5%
Purpose
Topical Analgesic
Uses
Temporary relief of aches and pains associated with, arthritis, carpal tunnel, swelling, sprains, bruising, inflammation
Warnings
For external use only
When Using This Product
Do not apply to wounds or irritated skin. Avoid contact with eyes, avoid use on deep wounds, avoid if you are allergic to listed ingredients.
Stop Use and consult a doctor if:
Condition worsens, Symptoms last for more than 7 days, condition clears up but occurs again in a few days, rash or irritation occurs.
If Pregnant of Breast-Feeding:
Consult a health professional before use
Directions
Dosage: Apply to affected area 1 to 4 times daily, or as needed.
Keep Out of Reach of Children
Keep Out of Reach of Children
Other Information
Store at 66-92 Degrees Fahrenheit.
Avoid direct sunlight.
Inactive Ingredients
Shea Butter, Jojoba Oil, Beeswax, Coconut Oil, Water, Vitamin E Oil, Magnesium Chloride, Cannabidiol Oil, Tetrahydrocannabinol Acetate, Maranta Arundinacea Root Powder
6 ounce jar label
MagneLeaf Pain Relief CBD THC Label
6 ounce lid label
 MagneLeaf THC Pain Relief Lid Label
MagneLeaf THC Pain Relief Lid Label
M J Green Enterprises, INC.

 MagneLeaf THC Pain Relief Lid Label
MagneLeaf THC Pain Relief Lid Label