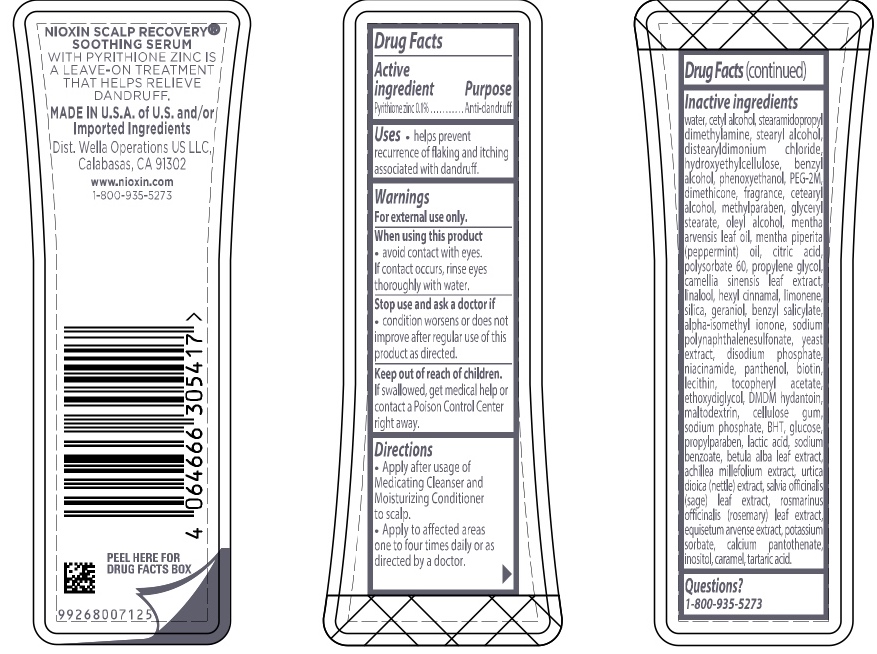
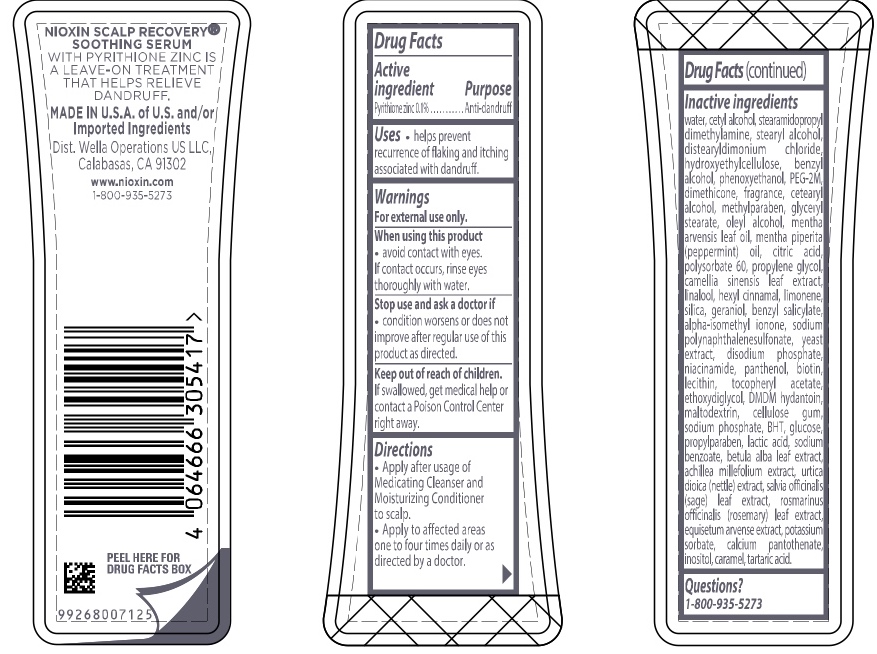
Label: NIOXIN SCALP RECOVERY SOOTHING SERUM- pyrithione zinc lotion
- NDC Code(s): 82157-005-10
- Packager: Wella Operations US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients water, cetyl alcohol, stearamidopropyl dimethylamine, stearyl alcohol, distearyldimonium chloride, hydroxyethylcellulose, benzyl alcohol, phenoxyethanol, PEG-2M, dimethicone, fragrance, cetearyl alcohol, methylparaben, glyceryl stearate, oleyl alcohol, mentha arvensis leaf oil, mentha piperita (peppermint) oil, citric acid, polysorbate 60, propylene glycol, camellia sinensis leaf extract, linalool, hexyl cinnamal, limonene, silica, geraniol, benzyl salicylate, alpha-isomethyl ionone, sodium polynaphthalenesulfonate, yeast extract, disodium phosphate, niacinamide, panthenol, biotin, lecithin, tocopheryl acetate, ethoxydiglycol, DMDM hydantoin, maltodextrin, cellulose gum, sodium phosphate, BHT, glucose, propylparaben, lactic acid, sodium benzoate, betula alba leaf extract, achillea millefolium extract, utica dioica (nettle) extract, salvia officinalis (sage) leaf extract, rosmarinus officinalis (rosemary) leaf extract, equisetum arvense extract, potassium sorbate, calcium pantothenate, inositol, caramel, tartaric acid
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIOXIN SCALP RECOVERY SOOTHING SERUM
pyrithione zinc lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82157-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength FORMALDEHYDE/SODIUM NAPHTHALENESULFONATE COPOLYMER (3000 MW) (UNII: 90D834OZUI) BENZYL SALICYLATE (UNII: WAO5MNK9TU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) URTICA DIOICA LEAF (UNII: X6M0DRN46Q) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) ROSEMARY (UNII: IJ67X351P9) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) WATER (UNII: 059QF0KO0R) STEARAMIDOPROPYL DIMETHYLAMINE (UNII: K7VEI00UFR) CETYL ALCOHOL (UNII: 936JST6JCN) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYETHYLENE OXIDE 100000 (UNII: V46Y6OJ5QB) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OLEYL ALCOHOL (UNII: 172F2WN8DV) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYSORBATE 60 (UNII: CAL22UVI4M) PEPPERMINT OIL (UNII: AV092KU4JH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM PHOSPHATE (UNII: SE337SVY37) BETULA PUBESCENS LEAF (UNII: 84SOH0O3OO) SODIUM BENZOATE (UNII: OJ245FE5EU) INOSITOL (UNII: 4L6452S749) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) BIOTIN (UNII: 6SO6U10H04) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) PANTHENOL (UNII: WV9CM0O67Z) NIACINAMIDE (UNII: 25X51I8RD4) LINALOOL, (+/-)- (UNII: D81QY6I88E) GERANIOL (UNII: L837108USY) DMDM HYDANTOIN (UNII: BYR0546TOW) MALTODEXTRIN (UNII: 7CVR7L4A2D) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) LACTIC ACID (UNII: 33X04XA5AT) TARTARIC ACID (UNII: W4888I119H) SAGE (UNII: 065C5D077J) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) CARAMEL (UNII: T9D99G2B1R) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82157-005-10 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 12/01/2022 Labeler - Wella Operations US LLC (117781338)