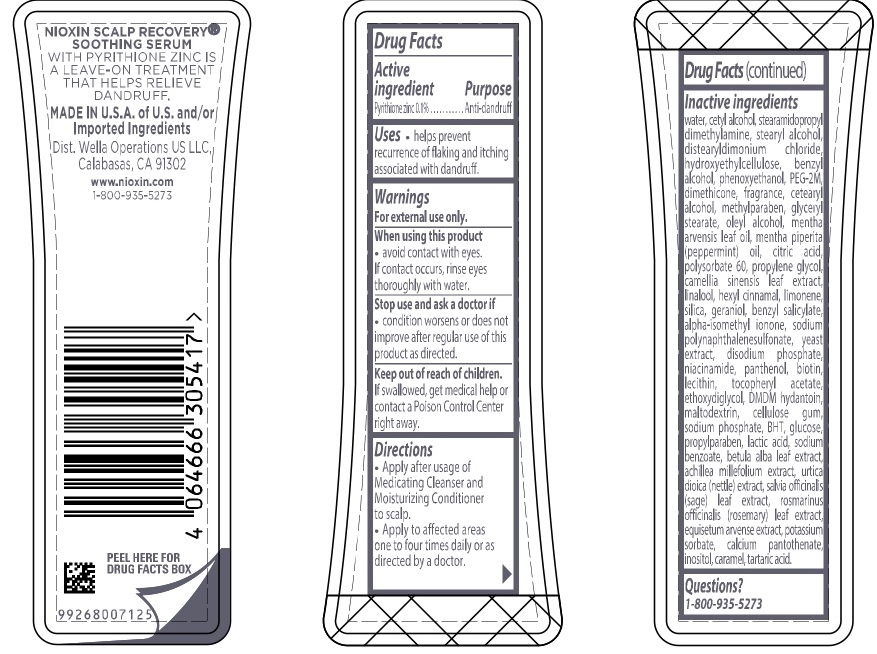
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Apply after usage of Medicating Cleanser and Moisturizing Conditioner to scalp.
- Apply to affected areas one to four times daily or as directed by a doctor.
Inactive ingredients water, cetyl alcohol, stearamidopropyl dimethylamine, stearyl alcohol, distearyldimonium chloride, hydroxyethylcellulose, benzyl alcohol, phenoxyethanol, PEG-2M, dimethicone, fragrance, cetearyl alcohol, methylparaben, glyceryl stearate, oleyl alcohol, mentha arvensis leaf oil, mentha piperita (peppermint) oil, citric acid, polysorbate 60, propylene glycol, camellia sinensis leaf extract, linalool, hexyl cinnamal, limonene, silica, geraniol, benzyl salicylate, alpha-isomethyl ionone, sodium polynaphthalenesulfonate, yeast extract, disodium phosphate, niacinamide, panthenol, biotin, lecithin, tocopheryl acetate, ethoxydiglycol, DMDM hydantoin, maltodextrin, cellulose gum, sodium phosphate, BHT, glucose, propylparaben, lactic acid, sodium benzoate, betula alba leaf extract, achillea millefolium extract, utica dioica (nettle) extract, salvia officinalis (sage) leaf extract, rosmarinus officinalis (rosemary) leaf extract, equisetum arvense extract, potassium sorbate, calcium pantothenate, inositol, caramel, tartaric acid