Label: MOUNTAIN SERIES WEEKENDER MEDICAL- benzalkonium chloride, povidone-iodine, acetaminophen, aspirin, diphenhydramine hydrochloride, ibuprofen, bacitracin zinc, neomycin sulfate, polymyxin b sulfate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 44224-0118-1, 47682-116-99, 47682-145-99, 47682-182-46, view more47682-808-99, 52124-0001-1, 52124-0003-1, 52380-0001-3 - Packager: Tender Corporation dba Adventure Medical Kits
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- Directions
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredient
-
PRINCIPAL DISPLAY PANEL
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic
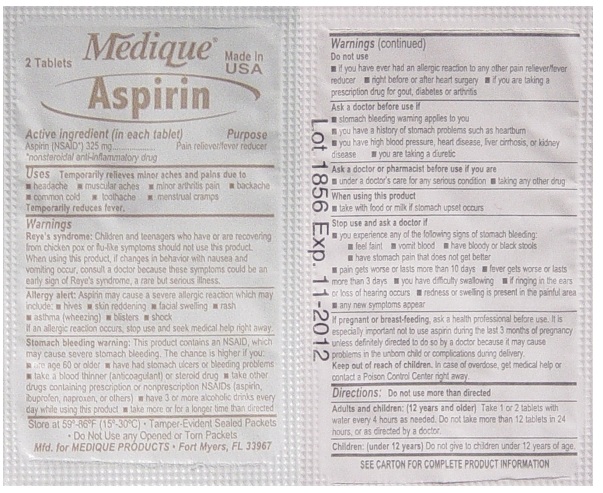
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription
NSAIDs (aspirin, ibuprofen, naproxen, or others) - have 3 or more alcohol drinks every day while
using this product - take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
- if you are taking prescription drugs for gout, diabetes or arthritis
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- if ringing in the ears or loss of hearing occurs redness or swelling is present in the painful area
- any new symptoms appear
-
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless
directed by a doctor (see Warnings) - drink a full glass of water with each dose
- Other information
- Inactive ingredients
- Questions or comments?
- 116R Medique APAP 325 mg Label
- Active ingredients
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking any drugs for asthma
- taking sedatives or tranquilizers
- Directions
- Other information
- Inactive ingredients
- Questions or comments? 1-800-634-7680
-
182R Medique Diphen Label
Collect Medi-Bucks
See inside flap for more details
Medique®
Diphen
Diphenhydramine HCl 25 mg
Hay Fever/Allergies
Fiebre del Heno/Alergias
Pull to Open
TiraParaAbrir
Easy To Swallow Capsules
Capsulas Faciles de Tragar
200 Capsules
(200 x 1)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Untarias
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- redness or swelling is present in the painful area
- any new or unexpected symptoms occur
- you experience any of the following signs of stomach bleeding:
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- do not use more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a doctor (see Warnings)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive ingredients
carnauba wax*, cellulose*, colloidal silicon dioxide, corn starch*, hypromellose, iron oxide red*, lactose, magnesium stearate, microcrystalline cellulose*, polydextrose, polyethylene glycol, povidone, silica*, sodium lauryl sulfate*, sodium starch glycolate, stearic acid*, titanium dioxide, triacetin*
*may contain
- QUESTIONS
- Principal Display Panel
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Stop using and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- redness or swelling is present
- Directions
- Inactive ingredients
- Questions or comments? 1-800-634-7680
- Principal Display Panel
-
145R Medique APAP 325 mg Label
Collect MediBucks
See inside flap for more details
Medique®
APAP
Acetaminophen 325 mg
Pain Reliever/Fever Reducer
Alivia el Dolor/Reduce la Fiebre
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
Tire Para Abrir
See new warnings information
500 Tablets
(250 x 2)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Unitarias
- Weekender Kit Label
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS AND PRECAUTIONS
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOUNTAIN SERIES WEEKENDER MEDICAL
benzalkonium chloride, povidone-iodine, acetaminophen, aspirin, diphenhydramine hydrochloride, ibuprofen, bacitracin zinc, neomycin sulfate, polymyxin b sulfate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44224-0118 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44224-0118-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 4.8 mL Part 2 3 TUBE 1.5 g Part 3 2 PACKET 4 Part 4 2 PACKET 2 Part 5 1 PACKET 2 Part 6 2 PACKET 4 Part 7 1 PACKET 22 g Part 1 of 7 ANTISEPTIC TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC:52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/23/2010 Part 2 of 7 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/16/2010 Part 3 of 7 MEDIQUE ASPIRIN
aspirin tablet, film coatedProduct Information Item Code (Source) NDC:47682-116 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 44;157;aspirin Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-116-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 12/30/2008 Part 4 of 7 MEDIQUE DIPHEN
diphenhydramine hydrochloride capsuleProduct Information Item Code (Source) NDC:47682-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink (pink) , white (white) Score no score Shape CAPSULE (CAPSULE) Size 14mm Flavor Imprint Code CPC;835 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-182-46 1 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/30/2008 Part 5 of 7 MEDI-FIRST IBUPROFEN
ibuprofen tablet, film coatedProduct Information Item Code (Source) NDC:47682-808 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-808-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075139 12/30/2008 Part 6 of 7 MEDIQUE APAP
acetaminophen tablet, film coatedProduct Information Item Code (Source) NDC:47682-145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-145-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 12/30/2008 Part 7 of 7 APLICARE POVIDONE-IODINE SOLUTION
povidone-iodine solution solutionProduct Information Item Code (Source) NDC:52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) POVIDONE-IODINE 9.8 g in 100 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52380-0001-3 22 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333A 08/01/2011 Labeler - Tender Corporation dba Adventure Medical Kits (064437304)