Use
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Keep out of reach of children: If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Antiseptic Towelette
Genuine First Aid LLC, Clearwater FL 33755
www.GenuineFirstAid.com
1/pouch
GENUINE FIRST AID
Active Ingredients
Active Ingredient: .........Bacitracin Zinc 400 units
Neomycin Sulfate 5mg ( equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units
Do not use: in eyes; over large areas of the body;
If allergic to any of the ingredients; for more than one week unless directed by a physician.
Stop use and consult a doctor:
if the condition persists or gets worse; a rash or other allergic reaction develops
Directions
Directions: clean affected area; apply small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily; may be covered with a sterile bandage
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic
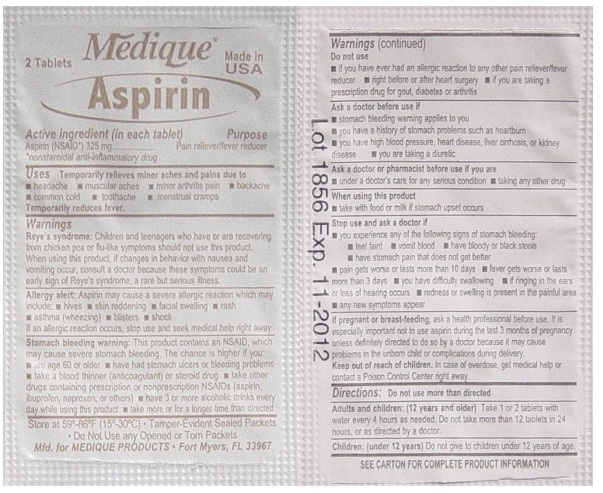
Uses
Temporarily relieves minor aches and pains associated with
- headache
- minor arthritis
- common cold
- menstrual cramps
- muscular aches
- backache
- toothache
Temporarily reduces fever.
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription
NSAIDs (aspirin, ibuprofen, naproxen, or others) - have 3 or more alcohol drinks every day while
using this product - take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
- if you are taking prescription drugs for gout, diabetes or arthritis
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- if ringing in the ears or loss of hearing occurs redness or swelling is present in the painful area
- any new symptoms appear
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless
directed by a doctor (see Warnings) - drink a full glass of water with each dose
Other information
- read all product information before using
- store at room temperature 59º-86ºF (15º-30ºC)
- avoid excessive heat and humidity
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
croscarmellose sodium*, hypromellose, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, propylene glycol*, starch, titanium dioxide*
*may contain
116R Medique APAP 325 mg Label
Medique®
Aspirin
5 Grain (325 mg)
Pain Reliever/Fever Reducer (NSAID)
See new warnings information
East To Swallow
Film Coated Tablets
24 Tablets
(12 x 2)
Compare active ingredients to Bayer®
Registered Trademark Bayer Consumer
Tamper Evident Unit Dose Packets
Uses
Temporarily relieves runny nose and decreases sneezing, itching of the nose and throat, itchy-watery eyes due to hay fever or other respiratory allergies.
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking any drugs for asthma
- taking sedatives or tranquilizers
Directions
- do not use more than directed
Other information
- store at room temperature 59º-86ºF (15º-30ºC)
- tamper-evident sealed packets
- do not use any opened or torn packets
182R Medique Diphen Label
Collect Medi-Bucks
See inside flap for more details
Medique®
Diphen
Diphenhydramine HCl 25 mg
Hay Fever/Allergies
Fiebre del Heno/Alergias
Pull to Open
TiraParaAbrir
Easy To Swallow Capsules
Capsulas Faciles de Tragar
200 Capsules
(200 x 1)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Untarias
Uses
Temporarily relieves minor aches and pains associated with
- ■
- headache
- ■
- backache
- ■
- common cold
- ■
- minor arthritis pain
- ■
- toothache
- ■
- menstrual cramps
- ■
- muscular aches
Temporarily reduces fever.
Allergy alert: Ibuprofen may cause a severe allergic reaction which may include:
- hives
- asthma (wheezing)
- rash
- blisters
- skin reddening
- facial swelling
- shock
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- redness or swelling is present in the painful area
- any new or unexpected symptoms occur
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless specifically directed to so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not use more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a doctor (see Warnings)
Adults and children: (12 years and older)
Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Other information
- read all product information before using
- store at 68-77°F (20-25°C)
- avoid excessive heat 40°C (above 104°F)
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
carnauba wax*, cellulose*, colloidal silicon dioxide, corn starch*, hypromellose, iron oxide red*, lactose, magnesium stearate, microcrystalline cellulose*, polydextrose, polyethylene glycol, povidone, silica*, sodium lauryl sulfate*, sodium starch glycolate, stearic acid*, titanium dioxide, triacetin*
*may contain
Principal Display Panel
Medi-First®
Ibuprofen 200 mg
Pain Reliever/Fever Reducer (NSAID)
Easy To Swallow Film Coated Tablets
See new warnings information
100 tablets (50 x 2)
Compare active ingredient to: Genuine Advil®
Registered trademark Wyeth Consumer
Tamper Evident Unit Dose Packets
Uses
For the temporary relief of minor aches and pains associated with
- headache
- common cold
- muscular aches
- toothache
- minor arthritis pain
- menstrual cramps
For the reduction of fever.
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Stop using and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- redness or swelling is present
Directions
- do not use more than directed
Inactive ingredients
corn starch, hypromellose, maltodextrin*, microcrystalline cellulose*, polyethylene glycol, povidone, pregelatinized starch*, sodium starch glycolate, stearic acid
* may contain
145R Medique APAP 325 mg Label
Collect MediBucks
See inside flap for more details
Medique®
APAP
Acetaminophen 325 mg
Pain Reliever/Fever Reducer
Alivia el Dolor/Reduce la Fiebre
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
Tire Para Abrir
See new warnings information
500 Tablets
(250 x 2)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Unitarias
Weekender Kit Label
Mountain Series Medical Kit Weekender
Adventure Medical Kits BE SAFE
1-6 People up to 7 days
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.