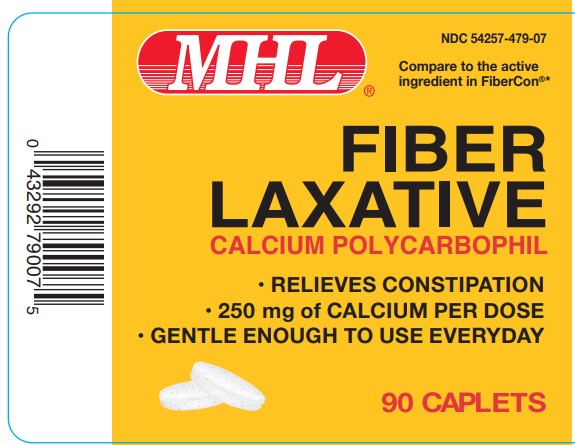
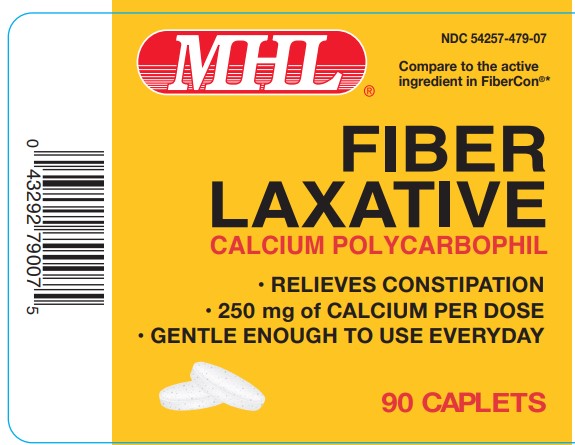
Label: FIBER LAXATIVE- calcium polycarbophil tablet
- NDC Code(s): 54257-479-07
- Packager: Magno-Humphries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Ask a doctor before use if you have
- abdominal pain, nausea, or vomiting
- a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product 2 or more hours before or after other drugs.
All laxatives may affect how other drugs work.When using this product
- do not use for more than 7 days unless directed by a doctor
- do not take more than 8 caplets in a 24 hour period unless directed by a doctor
Stop use and ask a doctor if rectal bleeding occurs or if you fail to have a bowel movement after use of this or any other laxative. These could be signs of a serious condition.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- take this product (child or adult dose) with a full glass of water (8 oz.) or other fluid. Taking this product without enough liquid may
cause choking. See choking warning.
- dosage will vary according to diet, exercise, previous laxative use or severity of constipation
- continued use for 1 to 3 days is normally required to provide full benefit
- adults and children 12 years of age and over: 2 caplets once a day up to 4 times a day
- children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FIBER LAXATIVE
calcium polycarbophil tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54257-479 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM POLYCARBOPHIL (UNII: 8F049NKY49) (POLYCARBOPHIL - UNII:W25LM17A4W) CALCIUM POLYCARBOPHIL 625 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) CROSPOVIDONE (UNII: 2S7830E561) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CARAMEL (UNII: T9D99G2B1R) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color white (Off-White) Score no score Shape OVAL (Caplet) Size 18mm Flavor Imprint Code RP120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54257-479-07 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 07/25/2022 Labeler - Magno-Humphries, Inc. (063251433)