Label: LEVULAN KERASTICK- aminolevulinic acid hydrochloride kit
- NDC Code(s): 67308-101-01, 67308-101-02, 67308-101-06
- Packager: Sun Pharmaceutical Industries, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LEVULAN ®KERASTICK ®safely and effectively. See full prescribing information for LEVULAN KERASTICK.
LEVULAN KERASTICK (aminolevulinic acid HCl) for Topical Solution, 20%
Initial U.S. Approval: 1999RECENT MAJOR CHANGES
INDICATIONS AND USAGE
LEVULAN KERASTICK for topical solution, a porphyrin precursor, plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is indicated for photodynamic therapy (treatment) of minimally to moderately thick actinic keratoses of the face or scalp, or actinic keratoses of the upper extremities. ( 1)
DOSAGE AND ADMINISTRATION
- LEVULAN KERASTICK photodynamic therapy is a two-stage process for administration by a health care provider.( 2.1)
- Apply the drug product to the target lesions. ( 2.1)
- Illuminate with blue light using the BLU-U
®Blue Light Photodynamic Therapy Illuminator after the incubation time of(
2.2):
- 14 to 18 hours for scalp or face
- 3 hours for upper extremities, with occlusion
- LEVULAN KERASTICK photodynamic therapy may be repeated a second time for lesions that have not completely resolved after 8 weeks ( 2.1)
- For topical use only. ( 2.1)
- See full prescribing information for complete dosage and administration instruction. ( 2)
- See BLU-U user manual for detailed lamp safety and operating instructions. ( 2.2)
DOSAGE FORMS AND STRENGTHS
After mixture, topical solution contains 20% aminolevulinic acid hydrochloride (ALA HCl) by weight in a plastic applicator device. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Transient amnestic episodes have been reported during postmarketing use of LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. Inform patients and their caregivers that LEVULAN KERASTICK in combination with PDT may cause transient amnestic episodes. Advise them to contact the healthcare provider if the patient develops amnesia after treatment. ( 5.1)
- Avoid exposure of the photosensitive actinic keratoses to sunlight or bright indoor light prior to blue light treatment. Protect treated lesions from sunlight exposure. Sunscreens will not protect the patient against photosensitivity reactions. ( 5.2)
- The LEVULAN KERASTICK for topical solution should be used by a qualified health professional. To avoid unintended photosensitivity, LEVULAN KERASTICK topical solution should be applied to no more than 5 mm of perilesional skin surrounding each target actinic keratosis lesion. ( 5.2)
- Irritation may be experienced if this product is applied to eyes or mucous membranes. Do not apply to the eyes or to mucous membranes. Excessive irritation may be experienced if this product is applied under occlusion longer than 3 hours. ( 5.3)
ADVERSE REACTIONS
The most common local adverse reactions (incidence ≥ 10%) were erythema, edema, stinging/burning, scaling/crusting, itching, erosion, hypo/hyperpigmentation, oozing/vesiculation/crusting, scaling and dryness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Dermatology at 877-533-3872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant use of other known photosensitizing agents such as St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulfonamides and tetracyclines might increase the photosensitivity reaction. ( 7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation and Administration Overview
2.2 Dosage and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Transient Amnestic Episodes
5.2 Photosensitivity
5.3 Irritation
5.4 Coagulation Defects
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
10.1 LEVULAN KERASTICK Topical Solution Overdose
10.2 BLU-U Light Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Actinic Keratoses of the Face or Scalp
14.2 Actinic Keratoses of the Upper Extremities
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Product Package - NDC Number
16.3 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation and Administration Overview
After mixing, the LEVULAN KERASTICK topical solution 20% is intended for direct application to individual lesions diagnosed as actinic keratoses and not to perilesional skin. This product is not intended for application by patients or unqualified medical personnel. Application should involve lesions on the scalp, face or upper extremities; multiple lesions can be treated within a treatment region, but multiple treatment regions should not be treated simultaneously.
The recommended treatment frequency is: one application of the LEVULAN KERASTICK topical solution and one dose of illumination per treatment region per 8-week treatment session. Each individual LEVULAN KERASTICK applicator should be used for only one patient.
LEVULAN KERASTICK photodynamic therapy for actinic keratoses is a two-stage process involving application of the LEVULAN KERASTICK topical solution to the target lesions and then illumination with blue light using the BLU-U Blue Light Photodynamic Therapy Illuminator after 3 hours for upper extremity lesions or after 14-18 hours for face or scalp lesions.
TABLE 1 Schedule for LEVULAN KERASTICK Photodynamic Therapy 1The incubation time is 14-18 hours for actinic keratosis lesions on the face or scalp.
2 The incubation time is 3 hours for actinic keratosis lesions on the upper extremities.LEVULAN KERASTICK topical solution application
Time window 1for Blue Light Illumination for face or scalp
Time window 2for Blue Light Illumination for upper extremities
6 am
8 pm to Midnight
9 am
7 am
9 pm to 1 am
10 am
8 am
10 pm to 2 am
11 am
9 am
11 pm to 3 am
12 Noon
10 am
Midnight to 4 am
1 pm
11 am
1 am to 5 am
2 pm
12 pm
2 am to 6 am
3 pm
1 pm
3 am to 7 am
4 pm
2 pm
4 am to 8 am
5 pm
3 pm
5 am to 9 am
6 pm
4 pm
6 am to 10 am
7 pm
5 pm
7 am to 11 am
8 pm
6 pm
8 am to Noon
9 pm
7 pm
9 am to 1 pm
10 pm
8 pm
10 am to 2 pm
11 pm
9 pm
11 am to 3 pm
12 Midnight
10 pm
Noon to 4 pm
1 am
If for any reason the patient cannot be given BLU-U Blue Light Photodynamic Therapy Illuminator treatment during the prescribed time after applying LEVULAN KERASTICK topical solution, he or she may nonetheless experience sensations of stinging and/or burning if the photosensitized actinic keratoses are exposed to sunlight or prolonged or intense light at that time. Advise the patient to wear appropriate protective apparel (e.g., wide-brimmed hat, long sleeve shirt, gloves) to shade the treated actinic keratoses from sunlight or other bright light sources until at least 40 hours after the application of LEVULAN KERASTICK topical solution. Advise the patient to reduce light exposure if the sensations of stinging and/or burning are experienced.
LEVULAN KERASTICK photodynamic therapy may be repeated a second time for lesions that have not completely resolved 8 weeks after the initial treatment.
2.2 Dosage and Administration Instructions
Step A - Treatment of Actinic Keratoses with LEVULAN KERASTICK Topical Solution
Preparation of Lesions
Actinic keratoses targeted for treatment should be clean and dry prior to application of LEVULAN KERASTICK Topical Solution.
Preparation of LEVULAN KERASTICK topical solution
The LEVULAN KERASTICK applicator consists of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle. The other ampule contains aminolevulinic acid HCl as a dry solid. LEVULAN KERASTICK topical solution is prepared by crushing the glass ampoules and mixing the contents together.
The LEVULAN KERASTICK topical solution can be prepared either manually, or using the optional KERASTICK KRUSHER™. These methods are illustrated below.
Figure 1: Manual Preparation:
- Hold the LEVULAN KERASTICK applicator with cap point up. Crush the bottom ampule containing the solution vehicle by applying finger pressure to Position A on the cardboard sleeve.
- Crush the top ampule containing the ALA HCl powder by applying finger pressure to Position B on the cardboard sleeve. To ensure both ampules are crushed continue crushing the applicator downward, applying finger pressure to Position A. Shake the LEVULAN KERASTICK applicator gently for at least 30 seconds to completely dissolve the drug powder in the solution vehicle.
Figure 2: Optional KERASTICK KRUSHER Preparation:
- Open the KERASTICK KRUSHER and properly position one LEVULAN KERASTICK applicator into the KRUSHER making sure to orient the LEVULAN KERASTICK label “A” with the KRUSHER “A”. Firmly seat the LEVULAN KERASTICK applicator against the closed end of the KRUSHER (cap should be at open end).
- Once positioned properly, close and firmly press the top and bottom handles together until the top and bottom handles touch one another along their length. A distinct crushing sound is made during this process. Ensure KRUSHER handles meet.
- Remove the LEVULAN KERASTICK applicator from the KRUSHER and shake the LEVULAN KERASTICK applicator gently for at least 30 seconds to completely dissolve the drug powder in the solution vehicle.
The LEVULAN KERASTICK topical solution must be used within two (2) hours of activation. If the solution is not completely applied within 2 hours of the activation, discard the applicator. If needed, use a new LEVULAN KERASTICK applicator.
Application of LEVULAN KERASTICK topical solution
Application of LEVULAN KERASTICK topical solution to Face or Scalp Lesions:
Following solution admixture, remove the cap from the LEVULAN KERASTICK applicator. The dry applicator tip should be dabbed on a gauze pad until uniformly wet with solution. Apply the solution directly to the target lesions by dabbing gently with the wet applicator tip. Enough solution should be applied to uniformly wet the lesion surface, including the edges without excess running or dripping. Once the initial application has dried, apply again in the same manner.
Do not apply the LEVULAN KERASTICK topical solution to the periorbital area or allow it to contact ocular or mucosal surfaces.
Photosensitization of the treated lesions will take place over the next 14-18 hours. The actinic keratoses should not be washed during this time. The patient should be advised to wear a wide-brimmed hat or other protective apparel to shade the treated actinic keratoses from sunlight or other bright light sources until BLU-U Blue Light Photodynamic Therapy Illuminator treatment. The patient should be advised to reduce light exposure if the sensations of stinging and/or burning are experienced.
At the visit for light illumination before treatment, the actinic keratoses treated with the LEVULAN KERASTICK topical solution should be gently rinsed with water and patted dry.
For Lesions on the Upper Extremities
Following solution mixture, remove the cap from the LEVULAN KERASTICK applicator. The dry applicator tip should be dabbed on a gauze pad until uniformly wet with solution. Apply the solution directly to the target lesions by dabbing gently with the wet applicator tip. Enough solution should be applied to uniformly wet the lesion surface, including the edges without excess running or dripping.
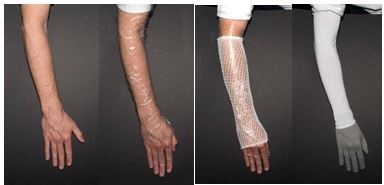
Occlude the upper extremity with low density polyethylene plastic wrap and hold in place with an elastic net dressing.
Figure 3: Method of Occlusion for Upper Extremities
The patient should wear a long-sleeved shirt and/or gloves or other protective apparel to shade the treated actinic keratoses from sunlight or other bright light sources until BLU-U Blue Light Photodynamic Therapy Illuminator treatment. Photosensitization of the treated lesions will take place over the next 3 hours. The actinic keratoses should not be washed during this time. Remove the occlusive dressing prior to light treatment and gently rinse the treated area(s) with water and pat dry before light illumination.
Step B - Administration of BLU-U Treatment:
LEVULAN KERASTICK is not intended for use with any device other than the BLU-U Blue Light Photodynamic Therapy Illuminator. Use of LEVULAN KERASTICK without subsequent BLU-U Blue Light Photodynamic Therapy Illuminator illumination is not recommended.
Photoactivation of actinic keratoses treated with LEVULAN KERASTICK topical solution is accomplished with illumination from the BLU-U Blue Light Photodynamic Therapy Illuminator. A 1000 second (16 minutes 40 seconds) exposure is required to provide a 10 J/cm 2light dose. During light treatment, both patients and medical personnel should be provided with blue blocking protective eyewear, as specified in the BLU-U Blue Light Photodynamic Therapy Illuminator Operating Instructions. Please refer to the BLU-U Blue Light Photodynamic Therapy Illuminator Operating Instructions for further information on conducting the light treatment. Patients should be advised that transient stinging and/or burning at the target lesion sites occurs during the period of light exposure.
If blue light treatment with the BLU-U Blue Light Photodynamic Therapy Illuminator is interrupted or stopped for any reason, it should not be restarted and the patient should be advised to protect the treated lesions from exposure to sunlight or prolonged or intense light for at least 40 hours after applying the LEVULAN KERASTICK topical solution.
For patients with facial lesions:
- The BLU-U Blue Light Photodynamic Therapy Illuminator is positioned so that the base is slightly above the patient’s shoulder, parallel to the patient’s face.
- The BLU-U is positioned around the patient’s head so the entire surface area to be treated lies between 2” and 4” from the BLU-U surface:
a) The patient’s nose should be no closer than 2” from the surface;
b) The patient’s forehead and cheeks should be no further than 4” from the surface;
c) The sides of the patient’s face and the patient’s ears should be no closer than 2” from the BLU-U surface.
For patients with scalp lesions:
- The knobs on either side of the BLU-U are loosened and the BLU-U is rotated to a horizontal position.
- The BLU-U is positioned around the patient’s head so the entire surface area to be treated lies between 2” and 4” from the BLU-U surface:
a) The patient’s scalp should be no closer than 2” from the surface;
b) The patient’s scalp should be no further than 4” from the surface;
c) The sides of the patient’s face and the patient’s ears should be no closer than 2” from the BLU-U surface.
For patients with upper extremity lesions:
-
The knobs on either side of the BLU-U Blue Light Photodynamic Therapy Illuminator are loosened and the light is rotated to a horizontal position.
- The BLU-U Blue Light Photodynamic Therapy Illuminator is positioned around the upper extremity so the entire surface area to be treated lies between 2” and 4” from the BLU-U surface. A table may be used to support the upper extremities during light treatment.
- Hold the LEVULAN KERASTICK applicator with cap point up. Crush the bottom ampule containing the solution vehicle by applying finger pressure to Position A on the cardboard sleeve.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
The LEVULAN KERASTICK for topical solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is contraindicated in patients with:
- Cutaneous photosensitivity at wavelengths of 400-450 nm [see Warnings and Precautions (5.2)]
- Porphyria or known allergies to porphyrins [see Warnings and Precautions (5.2)]
- Known sensitivity to any of the components of the LEVULAN KERASTICK.
-
5 WARNINGS AND PRECAUTIONS
5.1 Transient Amnestic Episodes
Transient amnestic episodes have been reported during postmarketing use of LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. Inform patients and their caregivers that LEVULAN KERASTICK in combination with PDT may cause transient amnestic episodes. Advise them to contact the healthcare provider if the patient develops amnesia after treatment.
5.2 Photosensitivity
After LEVULAN KERASTICK topical solution has been applied, the treatment site will become photosensitive and patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) for 40 hours. Exposure may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions.
Therefore, before exposure to sunlight, patients should protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material, and/or a long-sleeved shirt and/or gloves. Sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been determined if perspiration can spread the LEVULAN KERASTICK topical solution outside the treatment site to the eye or surrounding skin.
Application of LEVULAN KERASTICK topical solution to perilesional areas of photodamaged skin of the face, scalp or upper extremities may result in photosensitization. Upon exposure to activating light from the BLU-U, such photosensitized skin may produce a stinging and/or burning sensation and may become erythematous and/or edematous in a manner similar to that of actinic keratoses treated with LEVULAN KERASTICK Photodynamic Therapy. Because of the potential for skin to become photosensitized, the LEVULAN KERASTICK topical solution should be used by a qualified health professional to apply drug to no more than 5mm of perilesional skin surrounding the target actinic keratosis lesions.
If for any reason the patient cannot return for blue light treatment during the prescribed period after applying LEVULAN KERASTICK topical solution, the patient should call the doctor. The patient should also continue to avoid exposure of the photosensitized lesions to sunlight or prolonged or intense light for at least 40 hours. If stinging and/or burning is noted, exposure to light should be reduced.
5.3 Irritation
The LEVULAN KERASTICK topical solution contains alcohol and is intended for topical use only. Irritation may be experienced if this product is applied to eyes or mucous membranes. Do not apply to the eyes or to mucous membranes. Excessive irritation may be experienced if this product is applied under occlusion longer than 3 hours.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in the other sections of the labeling:
- Transient Amnestic Episodes [see Warnings and Precautions (5.1)]
- Increased Photosensitivity [see Warnings and Precautions (5.2)]
- Irritation [see Warnings and Precautions (5.3)]
- Coagulation defects [see Warnings and Precautions (5.4)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rate observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, no non-cutaneous adverse events were found to be consistently associated with LEVULAN KERASTICK photodynamic therapy.
Photodynamic Therapy Response:The constellation of transient local symptoms of stinging and/or burning, itching, erythema and edema as a result of LEVULAN KERASTICK photodynamic therapy (PDT) was observed in all clinical trials for actinic keratoses treatment. Stinging and/or burning subsided between 1 minute and 24 hours after the BLU-U Blue Light Photodynamic Therapy Illuminator was turned off, and appeared qualitatively similar to that perceived by patients with erythropoietic protoporphyria upon exposure to sunlight. There was no clear drug dose or light dose dependent change in the incidence or severity of stinging and/or burning.
Local skin reactions at the application site were observed in 99% of subjects treated with LEVULAN KERASTICK topical solution and BLU-U Blue Light Photodynamic Therapy Illuminator. The most common local adverse reactions (incidence ≥ 10%) were application site stinging/burning, erythema, edema, scaling/crusting, hypo/hyperpigmentation, itching, erosion, oozing/vesiculation/crusting, dryness.
In the trials for face and scalp lesions, severe stinging and/or burning at one or more lesions during light treatment was reported by at least 50% of subjects. Severe stinging and/or burning also occurred during light treatment in 9% of subjects receiving treatment for upper extremity lesions. The majority of subjects reported that all lesions treated exhibited at least slight stinging and/or burning. In trials of the face and scalp, the sensation of stinging/burning appeared to reach a plateau at 6 minutes into the treatment. Less than 3% of subjects receiving treatment for face or scalp lesions discontinued light treatment because of stinging/burning. No subjects discontinued light treatment in the trial for upper extremity lesions.
In trials for the face or scalp lesions, 99% of the active treatment group and 79% of the vehicle group experienced erythema shortly after treatment. In the trial for the upper extremity lesions, 99% of LEVULAN KERASTICK topical solution treatment group and 52% of the vehicle group experienced erythema on visit Days 2-3. Approximately 35% of LEVULAN KERASTICK topical solution group had edema, while edema occurred in <1% of the vehicle group. Both erythema and edema resolved to baseline or improved by 4 weeks after therapy for face or scalp. Edema resolved by 4 weeks and erythema resolved to baseline by 8 weeks for upper extremities.
The application of LEVULAN KERASTICK topical solution to perilesional skin resulted in stinging, burning, erythema and edema similar to treated actinic keratoses [see Warnings and Precautions (5.2)] .
Other Localized Cutaneous Adverse Experiences:Table 2depicts the incidence and severity of cutaneous adverse events in trials for the face and scalp.
TABLE 2 Post-PDT Cutaneous Adverse Events – ALA-018/ALA-019 For the Face and Scalp FACE
SCALP
LEVULAN KERASTICK Topical Solution + PDT (n=139)
Vehicle + PDT (n=41)
LEVULAN KERASTICK Topical Solution + PDT
(n=42)Vehicle + PDT (n=21)
Degree of Severity
Mild/ Moderate
Severe
Mild/ Moderate
Severe
Mild/ Moderate
Severe
Mild/ Moderate
Severe
Scaling/Crusting
71%
1%
12%
0%
64%
2%
19%
0%
Pain
1%
0%
0%
0%
0%
0%
0%
0%
Tenderness
1%
0%
0%
0%
2%
0%
0%
0%
Itching
25%
1%
7%
0%
14%
7%
19%
0%
Edema
1%
0%
0%
0%
0%
0%
0%
0%
Ulceration
4%
0%
0%
0%
2%
0%
0%
0%
Bleeding/Hemorrhage
4%
0%
0%
0%
2%
0%
0%
0%
Hypo/hyper-pigmentation
22%
20%
36%
33%
Vesiculation
4%
0%
0%
0%
5%
0%
0%
0%
Pustules
4%
0%
0%
0%
0%
0%
0%
0%
Oozing
1%
0%
0%
0%
0%
0%
0%
0%
Dysesthesia
2%
0%
0%
0%
0%
0%
0%
0%
Scabbing
2%
1%
0%
0%
0%
0%
0%
0%
Erosion
14%
1%
0%
0%
2%
0%
0%
0%
Excoriation
1%
0%
0%
0%
0%
0%
0%
0%
Wheal/Flare
7%
1%
0%
0%
2%
0%
0%
0%
Skin disorder NOS
5%
0%
0%
0%
12%
0%
5%
0%
Table 3depicts the incidence of other cutaneous adverse events in Phase 3 studies for the upper extremities.
TABLE 3 Percentage of Subjects with Cutaneous Adverse Reactions by the Most Severe Grade Reported Post-Baseline – CP0108 For Upper Extremities LEVULAN KERASTICK
Topical Solution + PDT
(N=135)Vehicle + PDT
(N=134)Degree of Severity
Minimal/
MildModerate/
SevereTotal
Minimal/
MildModerate/
SevereTotal
Edema
51%
4%
56%
7%
1%
8%
Erythema
35%
65%
100%
63%
12%
75%
Hyper-pigmentation
64%
9%
73%
57%
10%
66%
Hypo-pigmentation
46%
4%
50%
50%
5%
55%
Oozing/Vesiculation/ Crusting
36%
5%
41%
8%
2%
10%
Scaling and Dryness
65%
22%
87%
58%
7%
64%
Stinging/Burning
23%
73%
96%
23%
0%
23%
In the trial for upper extremity lesions, itching and scabbing occurred in 8% and 4%, respectively, of the subjects in the LEVULAN KERASTICK photodynamic therapy group. No subjects in the vehicle group reported itching or scabbing.
Common ( >2%, <10%) local cutaneous adverse reactions for face, scalp and upper extremities in the LEVULAN KERASTICK topical solution group included wheal, scabbing, pustules, ulceration, bleeding, tenderness and dysesthesia.
Uncommon (<2%) local cutaneous adverse reactions for face, scalp and upper extremities in the LEVULAN KERASTICK topical solution group were flaking, pain, peeling, perilesional pruritic rash, excoriation and blistering.
Common ( >2%, <10%) adverse reactions not limited to the application site for upper extremities and occurring more frequently in the LEVULAN KERASTICK topical solution group than in the vehicle group were sinusitis, squamous cell carcinoma, and squamous cell carcinoma of skin.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of LEVULAN KERASTICK. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous system disorders: transient amnestic episodes
-
7 DRUG INTERACTIONS
There have been no formal studies of the interaction of LEVULAN KERASTICK topical solution with any other drugs, and no drug-specific interactions were noted during any of the controlled clinical trials. It is, however, possible that concomitant use of other known photosensitizing agents such as St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulfonamides and tetracyclines might increase the photosensitivity reaction of actinic keratoses treated with LEVULAN KERASTICK topical solution [see Warnings and Precautions (5.2)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with LEVULAN KERASTICK topical solution use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. Animal developmental toxicology studies were not conducted with aminolevulinic acid. LEVULAN KERASTICK solution has low systemic absorption following topical administration, and the risk of maternal use resulting in fetal exposure to the drug is unknown [see Clinical Pharmacology (12.3)].
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of LEVULAN KERASTICK topical solution in either human or animal milk, the effects on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LEVULAN KERASTICK topical solution and any potential adverse effects on the breastfeeding child from LEVULAN KERASTICK topical solution or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients below the age of 18 have not been established. Actinic keratosis is not a disease generally seen in the pediatric population.
8.5 Geriatric Use
Of the 512 subjects in Phase 3 clinical trials of LEVULAN KERASTICK topical solution, 63% (321/512) were 65 years old and over, while 24% (123/512) were 75 years old and over. No overall differences in safety or substantial differences in effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
-
10 OVERDOSAGE
10.1 LEVULAN KERASTICK Topical Solution Overdose
In the event that the drug is ingested, monitoring and supportive care are recommended. The patient should be advised to avoid incidental exposure to intense light sources for at least 40 hours after ingestion. The consequences of exceeding the recommended topical dosage are unknown.
-
11 DESCRIPTION
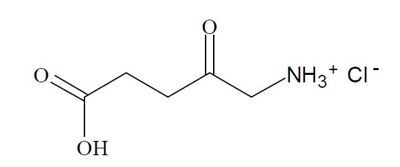
LEVULAN KERASTICK (aminolevulinic acid HCl) for topical solution, 20%, a porphyrin precursor, contains the hydrochloride salt of aminolevulinic acid (ALA), an endogenous 5-carbon aminoketone.
ALA HCl is a white to off-white, odorless crystalline solid that is very soluble in water, slightly soluble in methanol and ethanol, and practically insoluble in chloroform, hexane and mineral oil.
The chemical name for ALA HCl is 5-amino-4-oxopentanoic acid hydrochloride (MW = 167.59). The structural formula is represented below:
The LEVULAN KERASTICK for topical solution applicator is a two-component system consisting of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle comprising alcohol USP (ethanol content = 48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol. The other ampule contains 354 mg of aminolevulinic acid HCl as a dry solid. The applicator tube is enclosed in a protective cardboard sleeve and cap. The 20% topical solution is prepared just prior to the time of use by breaking the ampules and mixing the contents by shaking the LEVULAN KERASTICK applicator. “LEVULAN KERASTICK for topical solution” refers to the drug product in its unmixed state, “LEVULAN KERASTICK topical solution” refers to the mixed drug product (in the applicator tube or after application), and “LEVULAN KERASTICK” refers to the applicator only.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Following the application of LEVULAN KERASTICK topical solution, photosensitization occurs through the metabolic conversion of aminolevulinic acid to protoporphyrin IX (PpIX), a photosensitizer, which accumulates in the skin.
When exposed to light of appropriate wavelength and energy, the accumulated photoactive porphyrins produce a photodynamic reaction, resulting in a cytotoxic process dependent upon the simultaneous presence of oxygen. The absorption of light results in an excited state of porphyrin molecules, and subsequent spin transfer from photoreactive porphyrins to molecular oxygen generates singlet oxygen, which can further react to form superoxide and hydroxyl radicals. LEVULAN KERASTICK Photodynamic Therapy of actinic keratoses is the combination of photosensitization by application of the LEVULAN KERASTICK topical solution to the lesions and subsequent illumination with BLU-U Blue Light Photodynamic Therapy Illuminator.
12.2 Pharmacodynamics
ALA does not exhibit fluorescence, while PpIX has a high fluorescence yield. Time-dependent changes in surface fluorescence have been used to determine PpIX accumulation and clearance in actinic keratoses and perilesional skin after application of the LEVULAN KERASTICK topical solution in 12 subjects. Peak fluorescence intensity was reached in 11 ± 1 hr in actinic keratoses and 12 ± 1 hr in perilesional skin. The mean clearance half-life of fluorescence for lesions was 30 ± 10 hr and 28 ± 6 hr for perilesional skin. The fluorescence in perilesional skin was similar to that in actinic keratoses. Therefore, the LEVULAN KERASTICK topical solution should only be applied to the affected skin.
12.3 Pharmacokinetics
Two human pharmacokinetic (PK) studies were conducted in subjects with minimally to moderately thick actinic keratoses on the upper extremities, having at least 6 lesions on one upper extremity and at least 12 lesions on the other upper extremity. A single dose comprising of two topical applications of LEVULAN KERASTICK topical solution (each containing 354 mg ALA HCl) were directly applied to the lesions and occluded for 3 hours prior to light treatment.
The first PK study was conducted in 29 subjects and PK parameters of ALA were assessed. The baseline-corrected mean ± SD of the maximum concentration (C max) of ALA was 249.9 ± 694.5 ng/mL and the median time to reach C max(T max) was 2 hr post dose. The mean ± SD exposure to ALA, as expressed by area under the concentration time curve (AUC t) was 669.9 ± 1610 ng∙hr/mL. The mean ± SD elimination half-life (T 1/2) of ALA was 5.7 ± 3.9 hr.
A second PK study was conducted in 14 subjects and PK parameters of ALA and PpIX were measured. The baseline-corrected PpIX concentrations were negative in at least 50% of samples in 50% (7/14) subjects and AUC could not be estimated reliably. The baseline-corrected mean ± SD of C maxfor ALA and PpIX was 95.6 ± 120.6 ng/mL and 0.95 ± 0.71 ng/mL, respectively. The median T maxof ALA and PpIX was 2 hr post dose and 12 hr post dose, respectively. The mean AUC tof ALA was 261.1 ± 229.3 ng∙hr/mL. The mean ± SD T 1/2of ALA was 8.5 ± 6.7 hr.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity testing has been carried out using ALA. No evidence of mutagenic effects was seen in four studies conducted with ALA to evaluate this potential. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay (Ames mutagenicity assay), no increases in the number of revertants were observed with any of the tester strains. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay in the presence of solar light radiation (Ames mutagenicity assay with light), ALA did not cause an increase in the number of revertants per plate of any of the tester strains in the presence or absence of simulated solar light. In the L5178Y TK± mouse lymphoma forward mutation assay, ALA was evaluated as negative with and without metabolic activation under the study conditions. PpIX formation was not demonstrated in any of these in vitro studies. In the in vivo mouse micronucleus assay, ALA was considered negative under the study exposure conditions. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after ALA exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure.
No assessment of effects of ALA HCl on fertility has been performed in laboratory animals. It is unknown what effects systemic exposure to ALA HCl might have on fertility or reproductive function.
-
14 CLINICAL STUDIES
14.1 Actinic Keratoses of the Face or Scalp
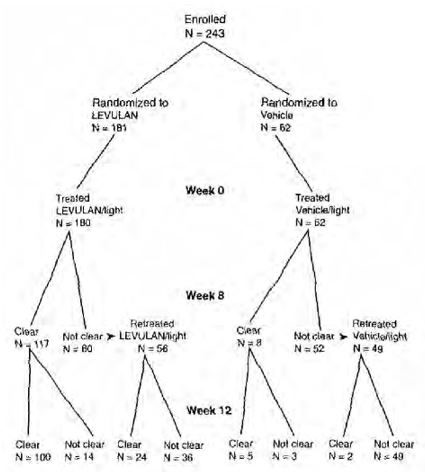
LEVULAN KERASTICK for topical solution plus blue light at 6-10.9 J/cm 2, has been used to treat actinic keratoses of the face or scalp in 342 subjects in seven clinical trials. Phase 3 trials ALA-018 and ALA-019 were two, identically designed, multicenter, two-arm trials using LEVULAN KERASTICK topical solution plus illumination from the BLU-U Blue Light Photodynamic Therapy Illuminator for 1000 seconds (16 minutes and 40 seconds) for a nominal exposure of 10 J/cm 2. Subjects were excluded from these trials who had a history of cutaneous photosensitization, porphyria, hypersensitivity to porphyrins, photodermatosis, or inherited or acquired coagulation defects. A minimum of 4 and a maximum of 15 clinically typical, discrete, Grade 1 (slightly palpable actinic keratoses: better felt than seen), or Grade 2 (moderately thick actinic keratoses: easily seen and felt) target actinic keratoses were identified (see Table 5for definitions). Target lesions on the face or on the scalp, but not on both locations in the same subject, received treatment. The subjects were randomized to receive treatment either with the LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator or vehicle plus BLU-U Blue Light Photodynamic Therapy Illuminator. Subjects were randomized at a 3 to 1 LEVULAN KERASTICK topical solution to vehicle ratio. A total of 243 subjects were enrolled in two Phase 3 trials (ALA-018, ALA-019). Lesions were designated as cleared (complete response) if the lesion had completely cleared and adherent scaling plaques of actinic keratoses were no longer evident on the surface of the treated skin when palpated. The percentage of subjects in whom 75% or more of treated lesions were cleared, and the percentage of subjects in whom 100% of treated lesions were cleared (Complete Responders), for each trial 8 weeks after treatment are shown in Table 4.
TABLE 4 - Subject Responses at Week 8 ALA-018
ALA-019
LEVULAN KERASTICK Topical Solution + PDT
Vehicle + PDT
LEVULAN KERASTICK Topical Solution + PDT
Vehicle + PDT
Subjects with >75% of AK Lesions Cleared
Total No. Subjects
68/87 (78%)
6/29 (21%)
71/93 (76%)
8/32 (25%)
Subjects with Face Lesions
57/71 (80%)
2/21 (10%)
57/67 (85%)
7/19 (37%)
Patients with Scalp Lesions
11/16 (69%)
4/8 (50%)
14/26 (54%)
1/13 (8%)
Complete Responders
Total No. Subjects
60/87 (69%)
4/29 (14%)
59/93 (63%)
4/32 (13%)
Subjects with Face Lesions
49/71 (69%)
2/21 (10%)
47/67 (70%)
4/19 (21%)
Subjects with Scalp Lesions
11/16 (69%)
2/8 (25%)
12/26 (46%)
0/13 (0%)
Because ALA-018 and ALA-019 had identical protocols, the combined results from the two trials are shown in the following tables. For actinic keratoses with a variety of thicknesses (excluding very thick, Grade 3 actinic keratoses which were not studied in the Phase 3 trials), LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator is more effective than vehicle plus BLU-U Blue Light Photodynamic Therapy Illuminator, but as shown in Table 5, the percentage of lesions with complete responses at 8 weeks after treatment with LEVULAN KERASTICK topical solution plus blue light illumination was lower for those lesions that were thicker at baseline. Efficacy of LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator on higher grade lesions was not studied in the Phase 3 clinical efficacy trials.
TABLE 5 Lesions Complete Responses at Week 8 for Different Lesion Grades Lesion Grade
LEVULAN
KERASTICK
Topical Solution + PDTVehicle + PDT
Grade 1
(slightly palpable actinic keratoses: better felt than seen)666/756 (88%)
122/302 (40%)
Grade 2
(moderately thick actinic keratoses: easily seen and felt)495/632 (78%)
52/199 (26%)
Grade 3
(very thick and/or hyperkeratotic actinic keratoses)0
0
Those subjects who were not Complete Responders at Week 8 had retreatment of the persistent target lesions at Week 8. Among the subjects undergoing retreatment, efficacy results seen at 12 weeks after the initial treatment, i.e., at 4 weeks after the second treatment, are shown in Table 6.
TABLE 6 Complete Responders at Week 12, Among Subjects Receiving Two Treatments LEVULAN KERATICK Topical Solution + PDT
Vehicle + PDT
Total No. Subjects
24/56 (43%)
2/49 (4%)
Subjects with Face Lesions
21/40 (53%)
2/31 (6%)
Subjects with Scalp Lesions
3/16 (19%)
0/18 (0%)
The efficacy results seen at 12 weeks after treatment, which include the results at 12 weeks for those subjects who received a single treatment as well as the results at 12 weeks for those subjects who received a second treatment at week 8, are shown in Table 7.
TABLE 7 Subject Responses at Week 12, Among Subjects Who Received One or Two Treatments LEVULAN KERASTICK
Topical Solution + PDTVehicle + PDT
Subjects with >75% of Actinic Keratosis Lesions Cleared
Total No. Subjects
158/180 (88%)
12/61 (20%)
Subjects with Face Lesions
127/138 (92%)
8/40 (20%)
Subjects with Scalp Lesions
31/42 (74%)
4/21 (19%)
Complete Responders
Total No. Subjects
129/180 (72%)
7/61 (11%)
Subjects with Face Lesions
108/138 (78%)
5/40 (13%)
Subjects with Scalp Lesions
21/42 (50%)
2/21 (10%)
Among Complete Responders at Week 8, 93% (in ALA-018) and 83% (in ALA-019) maintained complete response at Week 12. Among subjects with scalp lesions, the percentage of subjects with 100% of actinic keratosis lesions having complete response declined from Week 8 (55%) to Week 12 (50%), because there were more subjects with scalp lesions with 100% of actinic keratosis lesions cleared at Week 8 who had a recurrence of a lesion by Week 12 than there were subjects with scalp lesions who had retreatment of persistent lesions at Week 8 and who then achieved 100% of actinic keratosis lesions cleared by Week 12. Subjects did not receive follow-up past 12 weeks after the initial treatment.
Subject outcomes recorded in the two Phase 3 trials are depicted in the following flowchart, in which Complete Responders are designated clear. Seven subjects in the active treatment arm and three subjects in the vehicle treatment arm withdrew or were lost to follow-up. Three subjects in the active treatment arm were treated at baseline but did not return for evaluation until Week 12. One subject in the active treatment arm and two in the vehicle treatment arm who were not clear at Week 8 did not receive retreatment.
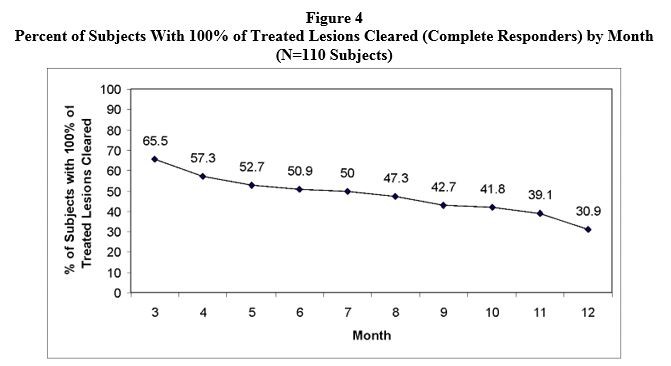
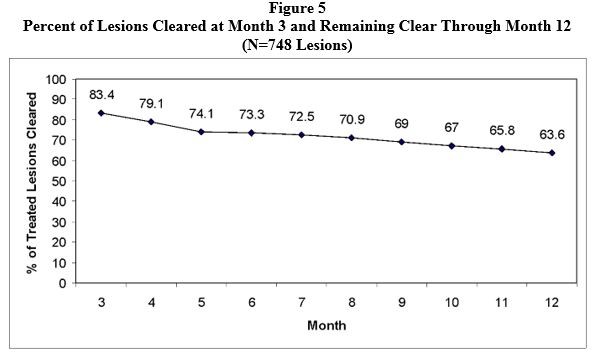
An open-label trial enrolled 110 subjects with 4 to 10 clinically typical, discrete actinic keratoses on the face or scalp, but not both locations. The target lesions were treated with the LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator. Any treated lesions that were not clear at Month 2 (Week 8) were re-treated. Subjects were followed monthly for 12 months. Lesions were designated as cleared if the lesion had completely cleared and adherent scaling plaques of actinic keratoses were no longer evident on the surface of the treated skin when palpated. The percentages of subjects in whom 100% of treated lesions were cleared (Complete Responders) by month, starting at Month 3 (Week 12), are shown in Figure 4. Of the 72 subjects with 100% of treated lesions cleared (Complete Responders) at Month 3, 53% had a recurrence by Month 12. A total of 748 individual lesions were treated; 539 were treated once and 209 were treated twice. At Month 3, 624 lesions (83%) were cleared. From Month 3 through Month 12 of the trial, 476 lesions (64%) remained clear. See Figure 5. Of the 624 treated lesions determined cleared at Month 3, 24% had recurred by Month 12, while 5% were lost to follow-up and their recurrence status is unknown.
14.2 Actinic Keratoses of the Upper Extremities
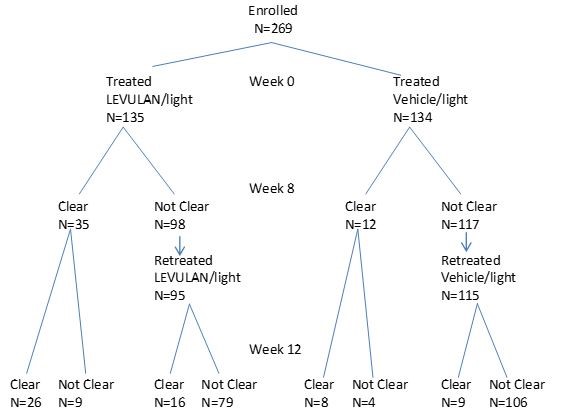
The safety and efficacy of LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator at 10J/cm 2to treat actinic keratoses of the upper extremities has been evaluated in a multicenter randomized, parallel-group, evaluator-blinded, vehicle-controlled trial of 269 subjects.
In this trial (CP0108), 269 subjects with 4 -15 mild to moderate actinic keratoses on the upper extremities (dorsal hand/forearm area between the elbow and the base of the fingers) were treated with LEVULAN KERASTICK topical solution and BLU-U Blue Light Photodynamic Therapy Illuminator. Subjects ranged from 45 to 90 years of age (mean 68 years) and 90% had Fitzpatrick Skin Type I, II or III. No subjects had Fitzpatrick Skin Type V or VI. Approximately 70% of subjects were male and all subjects were Caucasian.
Subjects were randomized to treatment in a 1:1 ratio to receive either LEVULAN KERASTICK topical solution or vehicle. Treatment was applied to 4-15 lesions on one dorsal hand/forearm on each subject, and occluded for the three-hour incubation period before treatment with 10 J/cm 2blue light delivered at 10 mW/cm 2. Treatment was repeated at Week 8 if any actinic keratosis lesions were present in the treatment area.
The primary endpoint was the proportion of subjects with complete clearance of all actinic keratosis lesions in the treatment area 12 weeks after initial treatment. The results of the trial are presented in Table 8.
Table 8 – Number and Percentage of Subjects with Actinic Keratosis of the Upper Extremities Achieving Complete Clearance at Week 12 LEVULAN KERASTICK
Topical Solution + PDTVehicle + PDT
CP0108
42/135 (31%)
17/134 (13%)
Subject outcomes recorded in the trial of the upper extremities are depicted in the following flowchart.
Subjects who achieved complete clearance at Week 12 entered a 12-month follow-up period. Subjects who received LEVULAN KERASTICK topical solution with blue light and achieved complete clearance at Week 12 in CP0108A had a recurrence rate of 58% (22/38) at 12 months, where recurrence was defined as the presence of at least one previously treated lesion in the treatment area at any visit during the 12-month follow-up period.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
The LEVULAN KERASTICK for topical solution, 20%, is supplied in packs of 6 applicators. Each LEVULAN KERASTICK applicator is for single use and consists of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle. The other ampule contains 354 mg of aminolevulinic acid HCl. The applicator is covered with a protective cardboard sleeve and cap.
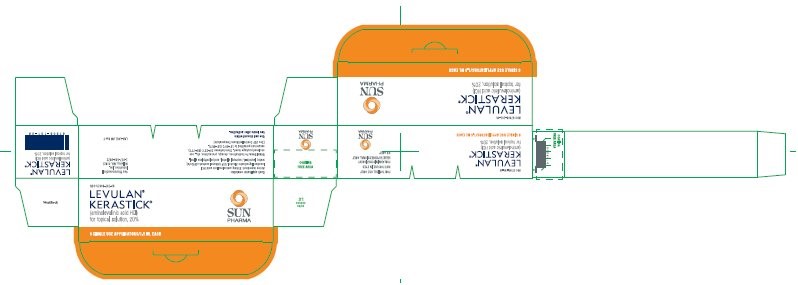
16.2 Product Package - NDC Number
Carton of 6 LEVULAN KERASTICK for topical solution, 20% applicators 67308-101-06
16.3 Storage
Store between 20° - 25 °C (68° - 77 °F); excursions permitted to 15°- 30 °C (59° - 86 °F) [See USP Controlled Room Temperature]. The LEVULAN KERASTICK topical solution should be used immediately following preparation (dissolution). Solution application must be completed within 2 hours of preparation. An applicator that has been prepared must be discarded 2 hours after mixing (dissolving) and a new LEVULAN KERASTICK applicator used, if needed.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Transient Amnestic Episodes
Transient episodes of amnesia have been reported with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. Advise patients and their families or caregivers to contact their healthcare provider if memory impairment, confusion, or disorientation is observed [see Warnings and Precautions (5.1)].
Photosensitivity
Transient episodes of amnesia have been reported with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. Advise patients and their families or caregivers to contact their healthcare provider if memory impairment, confusion, or disorientation is observed [see Warnings and Precautions (5.2)].
Advise patients that after LEVULAN KERASTICK topical solution has been applied, the treatment site will become photosensitive and that they should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) for 40 hours. Exposure may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions [see Warnings and Precautions (5.2)].
Advise patients to protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material, and/or a long-sleeved shirt and/or gloves. Advise patients sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been determined if perspiration can spread the LEVULAN KERASTICK topical solution outside the treatment site to the eye or surrounding skin [see Warnings and Precautions (5.2)].
If for any reason the patient cannot return for blue light treatment during the prescribed period after applying LEVULAN KERASTICK topical solution, advise patients to call the doctor. Advise patient to continue to avoid exposure of the photosensitized lesions to sunlight or prolonged or intense light for at least 40 hours. If stinging and/or burning is noted, exposure to light should be reduced [see Warnings and Precautions (5.2)].
Advise patients to avoid certain medications that may enhance the phototoxic reaction to PDT [see Drug Interactions (7)] .
Common Adverse Reactions
Inform patients that treatment with LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator may result in sensitivity to light, skin irritation and local skin reactions including erythema, edema, stinging/burning, scaling, crusting, oozing, vesiculation, wheal, scabbing, pustules, ulceration, itching, erosion, hypo/hyperpigmentation, bleeding, tenderness, dysesthesia, and dryness.LEVULAN, KERASTICK, KERASTICK KRUSHER, BLU-U, and DUSA are registered trademarks of DUSA Pharmaceuticals, Inc., a Sun Pharma company © 2020 Sun Pharmaceutical Industries, Inc. All rights reserved.
- Manufactured by: Sun Pharmaceutical Industries, Inc.
- Billerica, MA, 01821
- LAB-0530AW, Revision: F
Sun Pharmaceutical Industries, Inc.
Princeton, NJ
1-877-533-3872
-
SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL
LEVULAN ®KERASTICK ®
(aminolevulinic acid HCl) for Topical Solution, 20%
SINGLE USE APPLICATOR 1.5 mL
NDC 67308-101-01
A
↑
CRUSH
HERE
↓
A
LOT NO.:
EXP. DATE:
B
↑
CRUSH
HERE
↓
B
MANUFACTURED BY:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
LAB-0528 AW REV FNDC 67308-101-06
LEVULAN ®
KERASTICK ®
(aminolevulinic acid HCl)
for Topical Solution, 20%
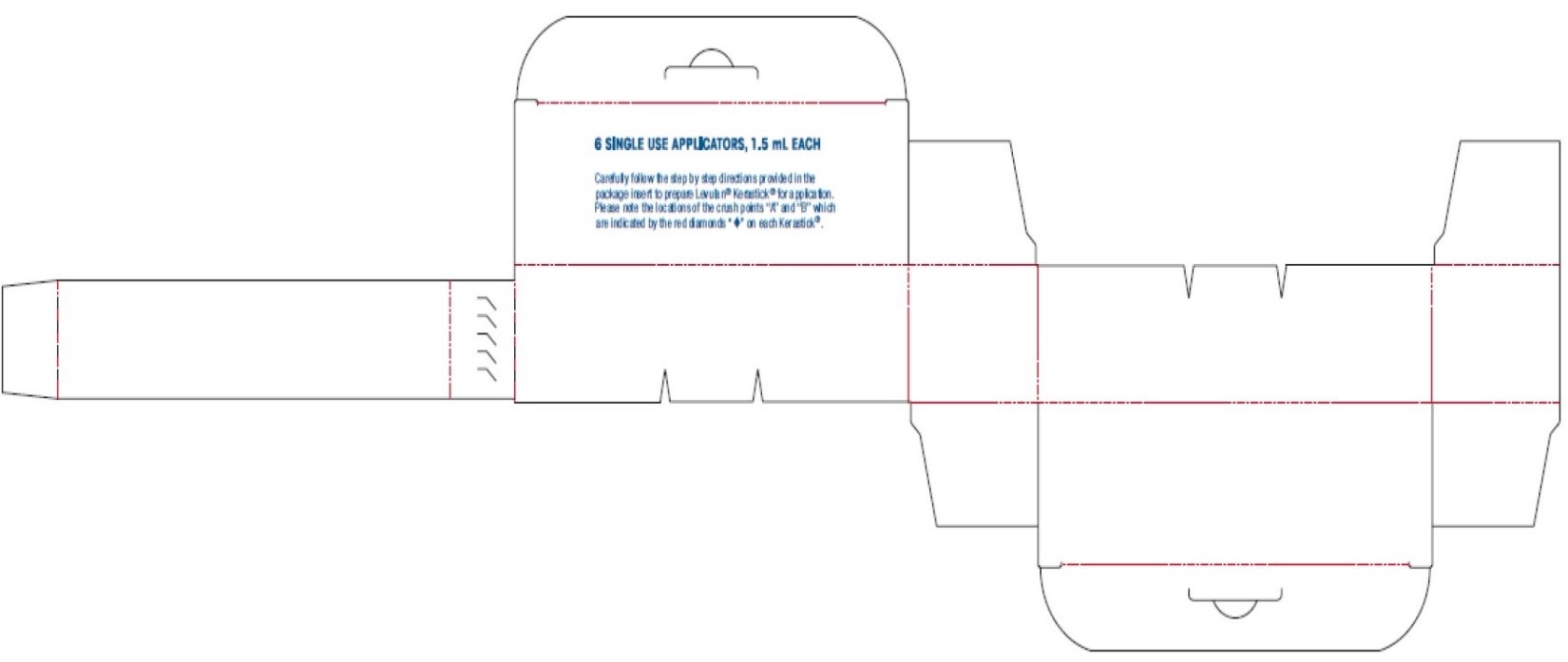
6 SINGLE USE APPLICATORS/1.5 ML EACH
FOR TOPICAL USE ONLY
NOT FOR USE IN EYES
FOR ADMINISTRATION BY HEALTH PROFESSIONAL ONLY
RX Only
Each applicator contains:
Active Ingredient: 354mg aminolevulinic acid HCl
Inactive Ingredients: Alcohol, USP (ethanol content=48%v/v), Water, laureth-4, isopropyl alcohol and polyethylene glycol.
Usual Dose:For indications, dosage, precautions, etc., see enclosed package insert.
Store between 20°– 25°C (68°-77°F), excursions permitted to 15°– 30°C (59° – 86°F).
[See USP Controlled Room Temperature]
Use and discard within two hours after activation.
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
1-877-533-3872 or
1-978-657-7500
LAB-1451 AW Rev F6 SINGLE USE APPLICATORS, 1.5 ML EACH
Carefully follow the step by step directions provided in the package insert to prepare Levulan ®Kerastick ®for application. Please note the locations of the crush points “A” and “B” which are indicated by the red diamonds “♦” on each Kerastick ®.NDC 67308-101-01
Levulan ®Kerastick ®
(aminolevulinic acid HCl) for Topical Solution, 20%
SINGLE USE APPLICATOR 1.5 ML
LOT NO.:
EXP. DATE:
SAMPLE: NOT FOR SALE
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
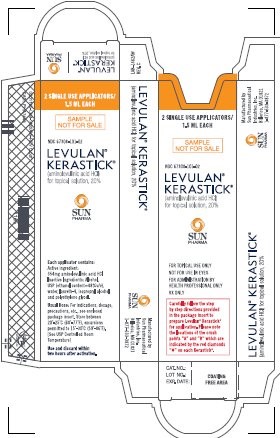
LAB-1411 REV.: CNDC 67308-101-02
Levulan® Kerastick ®
(aminolevulinic acid HCl) for Topical Solution, 20%
2 SINGLE USE APPLICATORS, 1.5 mL EACH
SAMPLE
NOT FOR SALE
For Topical Use Only
Not For Use in Eyes
FOR ADMINISTRATION BY HEALTH PROFESSIONAL ONLY
Rx only
Carefully follow the step by step directions provided in the package to prepare Levulan ®Kerastick ®for application. Please note the locations of the crush points "A" and "B" which are indicated by the red diamonds "♦" on each Kerastick ®.
CAT. NO.:
LOT NO.:
EXP. DATE:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
1-877-533-3872
LAB-1462AW REV. C
Each applicator contains:
Active Ingredient: 354mg aminolevulinic acid HCl
Inactive Ingredients: Alcohol, USP (ethanol content-48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol.
Usual Dose:
For indications, dosage, precautions, etc., see enclosed package insert.
Store between 20°25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature]
Use and discard within two hours after activation. -
INGREDIENTS AND APPEARANCE
LEVULAN KERASTICK
aminolevulinic acid hydrochloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67308-101 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67308-101-06 6 in 1 CARTON 09/24/2000 1 NDC:67308-101-01 1 in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:67308-101-02 2 in 1 CARTON 12/20/2000 2 NDC:67308-101-01 1 in 1 APPLICATOR; Type 0: Not a Combination Product 3 NDC:67308-101-01 1 in 1 APPLICATOR; Type 0: Not a Combination Product 09/04/2000 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 AMPULE 1.5 mL Part 2 1 AMPULE 1.5 mL Part 1 of 2 LEVULAN
aminolevulinic acid hydrochloride powder, for solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMINOLEVULINIC ACID HYDROCHLORIDE (UNII: V35KBM8JGR) (AMINOLEVULINIC ACID - UNII:88755TAZ87) AMINOLEVULINIC ACID HYDROCHLORIDE 354 mg in 1.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020965 09/24/2000 Part 2 of 2 DILUENT
diluent solutionProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) LAURETH-4 (UNII: 6HQ855798J) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020965 09/24/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020965 09/04/2000 Labeler - Sun Pharmaceutical Industries, Inc (139261648) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries, Inc 139261648 manufacture(67308-101)